Engineering of glycyrrhizin capped gold nanoparticles for liver targeting: in vitro evaluation and in vivo biodistribution study
Abstract
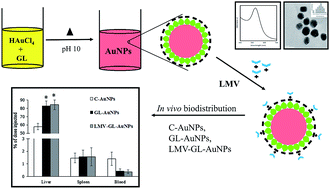
The purpose of this study was to explore the one pot green synthesis of non-cytotoxic gold nanoparticles (AuNPs) using glycyrrhizin as a reducing, stabilizing and targeting agent. The formation of glycyrrhizin (GL) capped gold nanoparticles was established by the presence of a surface plasmon resonance band at 523 nm. The as synthesized gold nanoparticles had a particle size of 17 ± 2 nm, with spherical shape and zeta potential value of −32 mV. GL-AuNPs showed excellent stability at varied pH, electrolyte conditions and cell culture media. The potential of synthesized gold nanoparticles as carrier of loaded antiviral drug lamivudine for liver targeting was explored. In vitro uptake studies by hepatocytes demonstrated a significant increase in cellular localization of GL-AuNPs as compared to citrate reduced gold nanoparticles (C-AuNPs). In vivo biodistribution study in Wistar rats proved the ability of GL-AuNPs for targeting liver. These finding can be utilized for site specific delivery of drugs for treatment of diseases affecting the liver.

 Please wait while we load your content...
Please wait while we load your content...