Synthesis, characterization and application of Ni0.5Zn0.5Fe2O4 nanoparticles for the one pot synthesis of triaryl-1H-imidazoles†
Ardeshir Khazaei*a,
Heidar Ali Alavi Nik*a,
Azam Ranjbarana and
Ahmad Reza Moosavi-Zareb
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan 6541835583, Iran. E-mail: Khazaei_1326@yahoo.com; Fax: +98-81-38380709; Tel: +98-81-38282807
bDepartment of Chemistry, Sayyed Jamaleddin Asadabadi University, Asadabad 6541835583, Iran
First published on 27th July 2016
Abstract
In this investigation, Ni0.5Zn0.5Fe2O4, as an efficient and heterogeneous catalyst, was prepared and characterized by IR, EDX, XRD and SEM analyses. Ni0.5Zn0.5Fe2O4 was successfully used for the synthesis of 2,4,5-triaryl substituted imidazoles by the one-pot, three component reaction under solvent-free conditions. Efficiency, generality, high yield, cleaner reaction profiles, ease of product isolation, short reaction times and simplicity are some advantages of this study.
In 1858, the synthesis of imidazole as an important compound was first reported by Debus.1 Imidazoles have various biological roles such as antithrombotic, anti-inflammatory,2 fungicidal,3 therapeutic,4 antitumor,8 and anti-HIV9 agents, as well as calcium antagonists; moreover, they act as inhibitors for P38 MAP kinase,5 B-Raf kinase,6 thromboxane A2 synthesase7 and HMG CoA reductase (HMGR).10 Imidazoles have been used in the synthesis of a large class of compounds such as ionic liquids.11–13
2,4,5-Triarylimidazoles were first synthesized from 1,2-dicarbonyl compounds, aldehydes and ammonia.14 Several procedures have been reported for the synthesis of triaryl-1H-imidazoles by the condensation reaction of α-hydroxyketones with ammonium acetate and various aromatic aldehydes in the presence of ionic liquids,15 HOAc,16 H2SO4,17 DMSO,18 H3PO4,19 silica sulfuric acid,20 sulfanilic acid,21 NiCl2·6H2O,22 iodine,23 CAN,24 NaHSO3,25 zeolite HY/silica gel,26 ZrCl4,27 tetrabutyl ammonium bromide,28 InCl3·H2O,29 KMnO4/CuSO4 (ref. 30) and microwave irradiation in presence of Al2O3 or HOAc.31 Some of these synthetic methods suffer from one or more drawbacks such as harsh reaction conditions, poor yields, laborious work-ups and purifications, prolonged reaction times, the use of expensive equipment (microwave or ultrasonic), corrosive reagents, and extensive acid catalysis. Recently, the use of reusable heterogeneous and solid acid catalysts have received considerable importance in organic synthesis due to several advantages, including operational simplicity, low toxicity, low cost, and ease of isolation after the completion of the reaction. The synthesis of magnetic nanoparticles as catalysts has gained considerable interest in recent years due to the magnetic properties of these particles, which leads to easy recovery and recycling of the catalyst.32
Nanoparticles such as CoFe2O4,33 ZnFe2O4,34 Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 with general ferrite formula (MFe2O4), are considered due to good magnetic properties, which M is one of the transition elements besides of Fe including Zn, Mn, Ni, Co. In addition, Ni–Zn ferrites are the most versatile magnetic materials as they have high saturation magnetization, high Curie temperature, chemical stability and relatively high permeability.32
35 with general ferrite formula (MFe2O4), are considered due to good magnetic properties, which M is one of the transition elements besides of Fe including Zn, Mn, Ni, Co. In addition, Ni–Zn ferrites are the most versatile magnetic materials as they have high saturation magnetization, high Curie temperature, chemical stability and relatively high permeability.32
Herein, we have reported a simple and efficient method for the synthesis of 2,4,5-triaryl substituted imidazoles using Ni0.5Zn0.5Fe2O4 as Fe-based metallic nanoparticles and catalysts with high yields, short reaction times, cleaner reaction profiles and ease of product isolation.
Results and discussion
According to previous literature studies,32b,c Ni0.5Zn0.5Fe2O4 nanoparticles as mixed metal oxides have been prepared and characterized using FT-IR, XRD, EDX and SEM analyses. The IR spectrum of Ni0.5Zn0.5Fe2O4 nanoparticles (Fig. 1) indicates that the prepared ferrite, with a spinel structure, has two principle metal–oxygen bands around 572 cm−1 related to stretching vibrations of the metal at the tetrahedral site (Fe–O). Moreover, the octahedral-metal stretching vibrations, related to Ni–O and Zn–O appear around 419 cm−1.36In another study, the X-ray diffraction (XRD) pattern of the catalyst was studied in a domain of 5–90° (Fig. 2) (JCPDS no. 08-0234). The XRD pattern displayed diffraction lines of a high crystalline nature at 2θ ≈ 30.00°, 35.50°, 43.00°, 53.50°, 57.10°, 62.5°, and several small lines in the 15–90° range, which is in good agreement with previous studies.36
To confirm the nanostructure of Ni0.5Zn0.5Fe2O4, TEM measurements were performed as showed in Fig. 3. The TEM micrograph confirms the presence of nanoparticles.
Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX) are widely used as surface analytical techniques. SEM of the catalyst clearly showed that the particles have not completely agglomerated and formed nanoparticles (Fig. 4). As shown in Fig. 4, the particles are 28–43 nm in size and have a spherical structure with only one type of particle morphology.
The elements in the Ni0.5Zn0.5Fe2O4 nanoparticles were studied by energy dispersive X-ray (EDX) spectrometry of the surface of the catalyst. The results of the EDX analysis of the Ni0.5Zn0.5Fe2O4 nanoparticles revealed the existence of Zn, Fe, Ni and O (Fig. 5). The actual chemical composition of the nanoparticles and the abundance of the elements were determined by ICPOES (inductively coupled plasma optical emission spectroscopy, Yvon-Jobin, France).
To optimize the reaction conditions, as a model reaction, a mixture of benzil (1 mmol), ammonium acetate (2.2 mmol), and benzaldehyde (1 mmol) was stirred in the presence of different amounts of Ni0.5Zn0.5Fe2O4 under different temperatures and solvent-free conditions. As seen in Table 1, 5 mol% of Ni0.5Zn0.5Fe2O4 was sufficient to catalyze the reaction at 80 °C under solvent-free conditions.
| Entry | Catalyst | Catalyst (mol%) | Temp (°C) | Time (min) | Yielda (%) | TOFb (min−1) | |
|---|---|---|---|---|---|---|---|
| a Isolated yield.b Turn over frequency. | |||||||
| 1 | Ni0.5Zn0.5Fe2O4 | 3 | 80 | 18 | 77 | 1.42 | |
| 2 | Ni0.5Zn0.5Fe2O4 | 4 | 80 | 12 | 81 | 1.68 | |
| 3 | Ni0.5Zn0.5Fe2O4 | 5 | 80 | 10 | 88 | 1.76 | |
| 4 | Ni0.5Zn0.5Fe2O4 | 6 | 80 | 10 | 88 | 1.46 | |
| 5 | Ni0.5Zn0.5Fe2O4 | 5 | 25 | 60 | 25 | 0.08 | |
| 6 | Ni0.5Zn0.5Fe2O4 | 5 | 60 | 35 | 60 | 0.34 | |
| 7 | Ni0.5Zn0.5Fe2O4 | 5 | 70 | 23 | 70 | 0.60 | |
| 8 | Ni0.5Zn0.5Fe2O4 | 5 | 80 | 10 | 80 | 1.60 | |
We also tested this reaction condition (5 mol% of Ni0.5Zn0.5Fe2O4) using different solvents. As shown in Table 2, solvent-free conditions are better than solvent-based conditions for the synthesis of 2,4,5-triaryl substituted imidazoles.
The catalytic activity of Ni0.5Zn0.5Fe2O4 nanoparticles as an efficient and heterogeneous catalyst was investigated in the synthesis of 2,4,5-triaryl-1H-imidazoles by the one-pot three component condensation reaction of ammonium acetate and aromatic aldehydes with various benzil compounds (Scheme 1 and Table 3).
To study different types of catalysts, we examined the model reaction in the presence of other Lewis acids, such as Fe3O4, ZnO and ZnFe2O4, in comparison with Ni0.5Zn0.5Fe2O4 (Table 4). As shown in Table 4, Ni0.5Zn0.5Fe2O4 is more successful than these catalysts for the synthesis of 2,4,5-triaryl substituted imidazoles.
The ease of recycling of the catalyst is one of the major advantages of this work. For this purpose, the reaction of benzil, ammonium acetate and benzaldehyde was tested with a recycled catalyst. We did not observe significant loss of the yields when Ni0.5Zn0.5Fe2O4 was reused for four runs. The results are depicted in Table 5.
Moreover, the results of this study were compared with the previously reported methods in Table 6. As shown in Table 6, our work is superior to some previous studies. The catalytic activity of Ni0.5Zn0.5Fe2O4 was improved by increasing the catalytic surface of the nanoparticle catalyst.
| Entry | Catalyst | Conditions | Time (min) | Yielda (%) [ref.] |
|---|---|---|---|---|
| a Isolated yield.b Our work. | ||||
| 1 | KH2PO4, (5 mol%) | Ethanol, reflux | 40 | 93 [37] |
| 2 | Yb(OPf)3 | C10F18, 80 °C | 360 | 80 [38] |
| 3 | [EMIM]OAc | EtOH, ultrasonic irradiation, r.t. | 45 | 87 [39] |
| 4 | InCl3·H2O | CH3OH, r.t. | 492 | 76 [40] |
| 6 | Ni0.5Zn0.5Fe2O4, (5 mol%) | Solvent free, 80 °C | 10 | 88b |
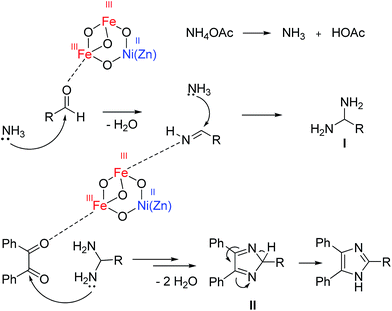
In a proposed mechanism supported by the literature,41,42 ammonia from NH4OAc attacks the carbonyl group of the activated aldehyde and produces 1,2-diamine (I). In the next step, by the reaction of 1,2-diamine with activated benzil along with the elimination of two molecules of H2O, II is prepared. A [1,5] hydrogen shift of II resulted in 2,4,5-triaryl imidazoles (Scheme 2).
Experimental
Synthesis of nano Ni0.5Zn0.5Fe2O4
In order to obtain the desired compositions, stoichiometric amounts of NiCl2·6H2O, ZnCl2 and FeCl3·6H2O were dissolved in ultra-pure water. The solutions of NiCl2·6H2O, ZnCl2 and FeCl3·6H2O in their stoichiometry (100 ml of 0.05 M NiCl2·6H2O, 100 ml of 0.2 M FeCl3·6H2O, 100 ml of 0.05 M ZnCl2) were dissolved in distilled water with a constant stirring. The neutralization was carried out with 1.5 M sodium hydroxide solution. The reaction temperature was kept at 85 °C for 45 min. The pH of the reaction was kept at 12. The precipitates were thoroughly washed with distilled water until the washings were free from sodium and chloride ions. The product was dried in an electric oven at a temperature of 120 °C for overnight to remove water contents. The dried powder was mixed homogeneously in a cleaned agate mortar and pestle.General procedure for the synthesis of 2,4,5-triaryl-1H-imidazoles
To a mixture of benzil (1 mmol), ammonium acetate (2.2 mmol) and aromatic aldehyde (1 mmol) in a test tube Ni0.5Zn0.5Fe2O4 nanoparticles (0.0118 g, 5 mol%) was added and the resulting mixture was stirred in an oil-bath at 80 °C for the appropriate time. After completion of the reaction, as monitored by TLC, ethanol was added and the mixture stirred for 5 min. The magnetic Ni0.5Zn0.5Fe2O4 nanoparticles were separated from the products using an external magnet and then washed twice with acetone, dried in a desiccator and stored for another subsequent reaction runs (the catalyst reused seven times in subsequent reaction without any significant changes in the yield and reaction times). The mixture was poured in cold water (50 ml), the precipitated solid was filtered, washed several times with water, dried and recrystallized from EtOH or acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (9
water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to get the corresponding 2,4,5-triaryl-1H-imidazoles. The pure products (compounds 1–11) were identified by IR, 1H, 13C NMR and mass spectra.
1) to get the corresponding 2,4,5-triaryl-1H-imidazoles. The pure products (compounds 1–11) were identified by IR, 1H, 13C NMR and mass spectra.
Conclusions
In this study, 2,4,5-triaryl-1H-imidazole has been synthesized in the presence of a catalytic amount of Ni0.5Zn0.5Fe2O4, as a recyclable and heterogeneous catalyst. Ni0.5Zn0.5Fe2O4 nanoparticles were synthesized and characterized by IR, EDX, XRD and SEM analyses. Green reaction conditions, good yields, easy work-up, short reaction times and efficiency of the catalyst are some of the advantages of this study.41,42Acknowledgements
We gratefully acknowledge the support of this work by the Research Council of Bu-Ali Sina University.Notes and references
- H. Debus, Liebigs Ann. Chem., 1858, 107, 199–208 CrossRef.
- J. G. Lombardino and E. H. Wiseman, J. Med. Chem., 1974, 17, 1182–1188 CrossRef CAS PubMed.
- A. F. Pozherskii, A. T. Soldatenkov and A. R. Katritzky, Heterocycles in Life Society, Wiley, NewYork, 1997 Search PubMed.
- J. Heeres, L. J. J. Backx, J. H. Mostmans and J. Vancustem, J. Med. Chem., 1979, 22, 1003–1005 CrossRef CAS PubMed.
- J. C. Lee, J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green and D. McNulty, Nature, 1994, 372–739, 739–746 CrossRef PubMed.
- A. K. Takle, M. J. B. Brown, S. Davies, D. K. Dean, G. Francis, A. Gaiba, A. W. Hird, F. D. King, P. J. Lovell, A. Naylor, A. D. Reith, J. G. Steadman and D. M. Wilson, Bioorg. Med. Chem. Lett., 2006, 16, 378–381 CrossRef CAS PubMed.
- P. Cozzi, G. Carganico, D. Fusar, M. Grossoni, M. Menichincheri, V. Pinciroli, R. Tonani, F. Vaghi and P. Salvati, J. Med. Chem., 1993, 36, 2964–2972 CrossRef CAS PubMed.
- L. Wang, K. W. Woods, Q. Li, K. J. Barr, R. W. McCroskey, S. M. Hannick, L. Gherke, R. B. Credo, Y. H. Hui, K. Marsh, R. Warner, J. Y. Lee, N. Zielinsky-Mozng, D. Frost, S. H. Rosenberg and H. L. Sham, J. Med. Chem., 2002, 45, 1697–1711 CrossRef CAS PubMed.
- C. Chuen, E. J. Bailey, H. David, F. H. David, L. H. Julie, G. A. I. Graham, S. J. Paul, E. K. Suzanne and E. K. Barrie, J. Med. Chem., 1996, 36, 3646–3657 CrossRef.
- P. Renukadevi, J. S. Biradar, S. P. Hiremath and S. Y. Manjunath, Indian J. Heterocycl. Chem., 1997, 6, 277–280 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed.
- S. Chowdhury, R. S. Mohan and J. L. Scott, Tetrahedron, 2007, 63, 2363–2389 CrossRef CAS.
- (a) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3691 CrossRef CAS PubMed; (b) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare and A. Zare, Org. Prep. Proced. Int., 2010, 42, 95–102 CrossRef CAS; (c) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare and A. Zare, J. Iran. Chem. Soc., 2010, 7, 646–651 CrossRef CAS; (d) A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare and A. Zare, Sci. Iran., Trans. C, 2010, 17, 31–36 CAS; (e) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, H. G. Kruger, Z. Asgari, V. Khakyzadeh and M. Kazem-Rostami, J. Org. Chem., 2012, 77, 3640–3645 CrossRef CAS PubMed; (f) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70–81 CrossRef CAS.
- B. Radziszewski, Chem. Ber., 1882, 15, 1493–1496 CrossRef.
- S. A. Siddiqui, U. C. Narkhede, S. S. Palimkar, T. Daniel, R. J. Lahoti and K. V. Srinivasan, Tetrahedron, 2005, 61, 3539–3546 CrossRef CAS.
- S. Sarshar, D. Siev and M. M. Mjalli, Tetrahedron Lett., 1996, 37, 835–838 CrossRef CAS.
- H. Weinmann, M. Harre, K. Koeing, E. Merten and U. Tilestam, Tetrahedron Lett., 2002, 43, 593–595 CrossRef CAS.
- N. G. Clark and E. Cawkill, Tetrahedron Lett., 1975, 16, 2717–2720 CrossRef.
- J. Liu, J. Chem, J. Zhao, Y. Zhao, L. Li and H. Zhang, Synthesis, 2003, 17, 2661–2666 Search PubMed.
- A. Shaabani and A. Rahmati, J. Mol. Catal. A: Chem., 2006, 249, 246–248 CrossRef CAS.
- A. F. Mohammed, N. D. Kokare, J. N. Sangshetti and D. B. Shinde, J. Korean Chem. Soc., 2007, 51, 418–422 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, H. A. Oskooie and S. Taheri, J. Mol. Catal. A: Chem., 2007, 263, 279–281 CrossRef CAS.
- M. Kidwai, P. Mothsra, V. Bansal and R. Goyal, Monatsh. Chem., 2006, 137, 1189–1194 CrossRef CAS.
- J. N. Sangshetti, N. D. Kokare, S. A. Kotharkara and D. B. A. Shinde, J. Chem. Sci., 2008, 120, 463–467 CrossRef CAS.
- J. N. Sangshetti, N. D. Kokare, S. A. Kothakar and D. B. Shinde, Monatsh. Chem., 2008, 139, 125–127 CrossRef CAS.
- S. Balalaie, A. Arabanian and M. Hashtroudi, Monatsh. Chem., 2000, 131, 945–948 CrossRef CAS.
- G. V. M. Sharma, Y. Jyothi and P. S. Lakshmi, Synth. Commun., 2006, 36, 2991–3000 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari and H. A. Oskooie, J. Mol. Catal. A: Chem., 2007, 263, 279–281 CrossRef CAS.
- S. D. Sharma, P. Hazarika and D. Konwar, Tetrahedron Lett., 2008, 49, 2216–2220 CrossRef.
- A. Khorramabadi-zad, M. Azadmanesh and S. Mohammadi, S. Afr. J. Chem., 2013, 66, 244–247 CAS.
- A. Y. Usyatinsky and Y. L. Khmelnitsky, Tetrahedron Lett., 2000, 41, 5031–5034 CrossRef CAS.
- (a) A. Goldman, Modern Ferrite Technology, Van Nostrand Reinhold, New York, 1990 Search PubMed; (b) H. T. Jiang, X. F. Wang and C. L. Yu, et al., Mater. Manuf. Processes, 2010, 25, 1489–1493 CrossRef CAS; (c) S. Dey, R. Gomes, R. Mondal, S. K. Dey, P. Dasgupta, A. Poddar, V. R. Reddy, A. Bhaumik and S. Kumar, RSC Adv., 2015, 5, 78508–78518 RSC.
- A. Chaudhuri, M. Mandal and K. Mandal, J. Alloys Compd., 2009, 487, 698–702 CrossRef CAS.
- (a) S. A. Shah, M. U. Hashmi, S. Alam and A. Shamim, J. Magn. Magn. Mater., 2010, 322, 375–381 CrossRef CAS; (b) A. Khazaei, A. Ranjbaran, F. Abbasi, M. Khazaei and A. R. Moosavi-Zare, RSC Adv., 2015, 5, 13643–13647 RSC.
- (a) H. M. Fan, J. B. Yi and Y. Yang, ACS Nano, 2009, 3, 2798–2808 CrossRef CAS PubMed; (b) A. Khazaei, A. R. Moosavi-Zare, H. Afshar-Hezarkhani and V. Khakyzadeh, RSC Adv., 2014, 4, 32142–32147 RSC; (c) M. A. Zolfigol, A. R. Moosavi-Zare, P. Moosavi, V. Khakyzadeh and A. Zare, C. R. Chim., 2013, 16, 962–966 CrossRef CAS; (d) M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Zare, P. Arghavani-Hadi, Z. Mohammadi and M. H. Beyzavi, S. Afr. J. Chem., 2012, 65, 280–285 CAS.
- N. M. Deraza and O. H. Abd-Elkaderb, J. Anal. Appl. Pyrolysis, 2014, 106, 171–176 CrossRef CAS; K. H. Wu, Y. M. Shin, C. C. Yang, W. D. Ho and J. S. Hsu, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2657–2664 CrossRef.
- R. S. Joshi, P. G. Mandhane, M. U. Shaikh, R. P. Kale and C. H. Gill, Chin. Chem. Lett., 2010, 21, 429–432 CrossRef CAS.
- M. Shen, C. Cai and W. Yi, J. Fluorine Chem., 2008, 129, 541–544 CrossRef CAS.
- H. Zang, Q. Su, Y. Mo, B. W. Cheng and S. Jun, Ultrason. Sonochem., 2010, 17, 749–751 CrossRef CAS PubMed.
- S. D. Sharma, P. Hazarika and D. Konwar, Tetrahedron Lett., 2008, 49, 2216–2220 CrossRef.
- M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Zare, P. Arghavani-Hadi, Z. Mohammadi and M. H. Beyzavi, S. Afr. J. Chem., 2012, 65, 280–285 CAS.
- K. F. Shelke, S. B. Sapkal, S. S. Sonar, B. R. Madje, B. B. Shingate and M. S. Shingare, Bull. Korean Chem. Soc., 2009, 30, 1057–1060 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Including H NMR and C NMR spectral data. See DOI: 10.1039/c6ra05158h |
| This journal is © The Royal Society of Chemistry 2016 |