Self-assembled fibrillar networks comprised of a naturally-occurring cyclic peptide—LOB3†
Abstract
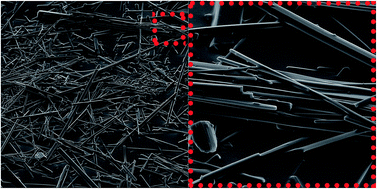
To the best of our knowledge, this is the first report of a self-assembling orbitide that is capable of forming 1D nano-fibers and ultimately 3D molecular gel networks. LOB3 (a.k.a. cyclolinopeptide A), extracted from Linum usitatissimum L. (flaxseed), forms molecular gels in acetonitrile. LOB3 molecular gels, illustrate that cyclic peptides may be comprised of more complex amino acid sequences than have been currently reported. It appears that cyclization, to form orbitides, imparts conformational aspects to the molecule facilitating self-organization into crystalline nano-fibers. These nanoscale fibers, ∼300 nm in diameter and >100 μm in length, aggregate into bundles of fibers which may exceed micron dimensions. Within the nano-fibers, the orbitides adapt an antiparallel β-sheet-like conformation with high molecular periodicity, as illustrated by CD and XRD.

 Please wait while we load your content...
Please wait while we load your content...