Influence of material characteristics on the structure and properties of high-density polyethylene microporous membranes
Abstract
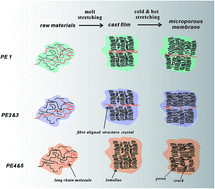
Five PE resins with different material characteristics were chosen to fabricate microporous membranes based on a melt-stretching mechanism. The lamellar morphology of the initial precursor films and stretched pore structures were characterized. It was found that the precursor films prepared using PE with an appropriate content of larger and small molecular weight species showed higher lamellar thickness and orientation degree. This kind of PE resins exhibited a weak relaxation peak on the left of the main relaxation peak in the relaxation time curves. After stretching, a porosity of around 50% could be obtained and the corresponding air permeability could meet the demands of a separator, which could be used in the field of lithium batteries. Contrary to this, a PE with too many larger molecular weight species was unfavorable for the preparation of microporous membranes, since the higher content of fibre-aligned structures limited the separation of lamellar structures during stretching. PE with too low a content of small molecular weight parts was also inapplicable for the preparation of microporous membranes. The lower lamellar thickness was easy to be deformed and could not support the scaffold of the pore structure, leading to the collapse of the pores and inferior air permeability.

 Please wait while we load your content...
Please wait while we load your content...