Multi-target detection of oxidative stress biomarkers in quercetin and myricetin treated human red blood cells
Pawan Kumar Maurya†
*ab,
Prabhanshu Kumar†a,
Shirisha Nagotuc,
Subhash Chandd and
Pranjal Chandra*c
aAmity Institute of Biotechnology, Amity University Uttar Pradesh, Noida, 201301, India. E-mail: pkmaurya@amity.edu; Fax: +91 120 2432200; Tel: +91 9560869477
bInterdisciplinary Laboratory for Clinical Neuroscience (LiNC), Department of Psychiatry, Universidade Federal de Sao Paulo – UNIFESP, Brazil
cDepartment of Biosciences and Bioengineering, Indian Institute of Technology-Guwahati, Guwahati-781 039, Assam, India. E-mail: pchandra13@iitg.ernet.in; Fax: +91-361-258-2249; Tel: +91-361-258-3207
dDepartment of Biochemical Engineering & Biotechnology, Indian Institute of Technology, Delhi, India
First published on 25th May 2016
Abstract
Quercetin and myricetin are important dietary flavonoids with potential health benefits and interfere with reactive oxygen species metabolism. The objective of this study was multi-target spectroscopic analysis of oxidative stress biomarkers (malondialdehyde (MDA), glutathione (GSH) and sulfhydryl (–SH) groups) in quercetin and myricetin treated red blood cells (RBCs) during human aging. The study was carried out on clinically relevant blood samples obtained from 105 healthy subjects between the ages of 18–82 years. The subjects were divided into three age groups, young (18–35 years), middle (36–60 years) and old (>60 years). Oxidative stress was induced in vitro by incubating RBCs with 10−5 M tert-butyl hydroperoxide (t-BHP). The effects of flavonoids were evaluated by detecting MDA, GSH and –SH groups by co-incubating the RBCs in the presence of flavonoid (10−8 M to 10−5 M final concentration) and t-BHP. The GSH/GSSG ratios were estimated to demonstrate the antioxidant power of the RBCs. The results showed elevated MDA levels (p < 0.001) after incubation with t-BHP as compared to a control. The GSH and –SH groups significantly (p < 0.001) decreased when incubated with t-BHP. In vitro administration of both flavonoids significantly attenuated the deleterious effect of oxidative stress in erythrocytes from all age groups. We showed a significant (p < 0.0001) negative correlation (r = −0.8334) between GSH/GSSG during human aging. We believe that these findings are novel and will help in the fast screening of new chemical molecules which may help against oxidative stress in RBCs, and thereby have tremendous scope in medical diagnostics and therapeutics.
Introduction
The role of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in aging has been extensively investigated in several cellular and animal models and various connections have been established.1 On the one hand, appropriate levels of ROS/RNS play an important role in the modulation of several physiological responses, as ROS/RNS are part of a signalling network regulating cell function. On the other hand, excessive intracellular levels of ROS/RNS, as well as a defective antioxidant system, can give rise to pathological conditions and contribute to the etiology of diverse human diseases, such as coronary heart disease, diabetes, and hypertension.2,3 Aging leads to the accumulation of diverse detrimental changes in cells and tissues, resulting in an increased risk of disease and death.1 There are more than three hundred theories that attempt to explain the process of aging. The oxidative stress hypothesis offers the best mechanistic explanation of aging and age-related phenomenon.4 In aerobic respiration, the univalent reduction of molecular oxygen results in the generation of ROS including superoxide anion (O2−˙), hydroxyl radicals (˙OH) as well as hydrogen peroxide (H2O2).5 Excessive ROS damages carbohydrates, proteins, lipids, and nucleic acids (DNA & RNA), thus leading to aging and age related diseases.6 However, aerobic organisms have antioxidant defence system that protects against oxidative stress induced injury. This defence system includes the enzymatic systems like superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST).7–9 ROS causes oxidative injury when antioxidant defence of the body is overwhelmed. A certain amount of oxidative damage takes place even under normal conditions, however, the rate of this damage increases during aging as the efficiency of antioxidative and repair mechanisms decrease.10,11 Measure of oxidative stress in red blood cells and their implications in tissue damage, disease has been shown,12–14 but report on changes of oxidative stress markers in red blood cells during human aging has been rarely attempted.Red blood cells (RBCs) are continuously exposed to oxygen tension and its membrane is rich in polyunsaturated fatty acids (PUFA) and proteins which are easy target of ROS.15 RBCs have evolved mechanism to protect themselves from intrinsic oxidative stress and have developed a sophisticated adaptation system that essentially involves the rearrangement of the antioxidant function. Lipids are susceptible to oxidative modifications. Lipid peroxidation leads to the formation of lipid radicals and several low molecular weight decomposition products, such as acrolein, malondialdehyde, and 4-hydroxy-2-nonenal, that are highly reactive towards proteins, DNA, and phospholipids.16 These aldehydic molecules mediate toxic effects both in cell and animal models. Glutathione is the major non-enzymatic component of intracellular antioxidant defenses. It is present at millimolar concentration, and it works either as a nucleophile for efficient detoxification of reactive electrophiles, or as an antioxidant. Glutathione is present in a reduced, biological active form (GSH), and in an oxidised form, namely glutathione disulphide (GSSG).17 Membrane sulfhydryl (–SH) group also provide protection against ROS/RNS.
The persistent pro-oxidative state characterizing aging cells, as well as their multiple adaptation mechanism, can be exploited to develop new therapeutic strategies. Diets rich in fruits and vegetables are associated with reduced risk of aging and age associated disorders. Dietary components of fruits and vegetables also act as important protective agents against age associated damage.18 They belong to the flavonoid group of plant metabolites. Flavonoids are structurally heterogeneous polyphenolic compounds, which are widely distributed in plant food, and which may exert beneficial effects including protection from cardiovascular disease, cancer, diabetes, hypertension, and neurodegenerative disorders.19 Most of the health benefits originate from their potent antioxidant and free radical scavenging properties, as well as their ability to modulate many metabolic functions.20 Flavonoids have multiple biological effects both in vivo and in vitro. They are antioxidants, anti-inflammatory, antiviral, anti-allergic agents that can modulate a wide range of mammalian enzyme activities, such as cytochrome P450, epoxide hydratase, glutathione transferase, DNA and RNA polymerase, and topoisomerase.21
Quercetin (3,3′,4′,5′,7-pentahydroxyflavone) and myricetin (3,3′,4′,5′,5′,7-hexahydroxylflavone) are natural flavonoids found in many fruits, vegetables, herbs and other plants (Fig. 1). They are the most abundant dietary flavonoids found in onion, red grapes, apples, tea, capers, oak tree, citrus fruits, pepper, lovage, broccoli, cherries and berries.22,23 They are known as potent natural antioxidants and scavengers of ROS/RNS under in vitro and in vivo conditions. Besides antioxidant properties, they show cyto-protective, anti-inflammatory, and anti-mutagenic effects.24,25 Controversial studies about the health benefits of these flavonoids are reported in literature. Certain studies support its cardio-protective and anticancer activities, others fail to support this relation.26 The biological process of aging can be influenced by environmental factors, such as natural antioxidants, pollution, etc. A large number of studies on these flavonoids and their different targets of cell/tissue are available, but very few studies on aging are reported in literature and in human cells. In our earlier studies, we have been successful in elucidating protective effect of dietary flavonoids on biomarkers of oxidative stress during aging in humans.27–29 Encouraged by these results, we tried to investigate the effect of potent, natural antioxidant i.e. quercetin and myricetin, on biomarkers of oxidative stress during human aging.
Human red blood cells serve as a good model to study the effect of oxidative stress on metabolism.30 There is no complication in interpreting metabolic data due to changes in transcription and translation. Therefore, we investigate effect of flavonoids to healthy aging and comparing their effect in different age populations. To examine the effect of quercetin and myricetin, we used in vitro model and spectroscopic analysis for multi-target detection of oxidative stress biomarkers as shown in Scheme 1.
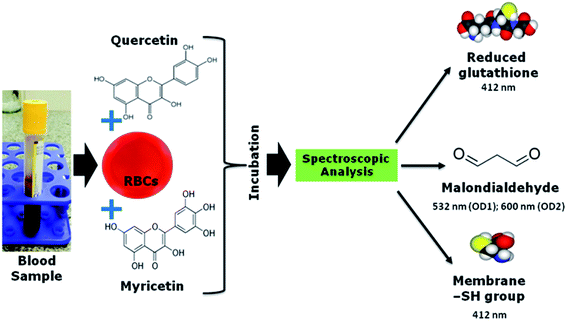 | ||
| Scheme 1 Schematic representation of methodology adapted for analysis of oxidative stress biomarker in blood sample. | ||
Results and discussion
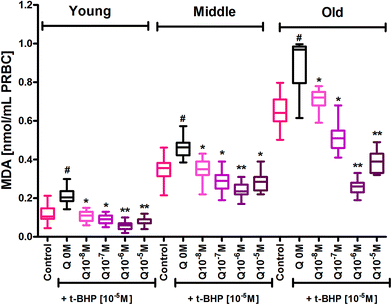
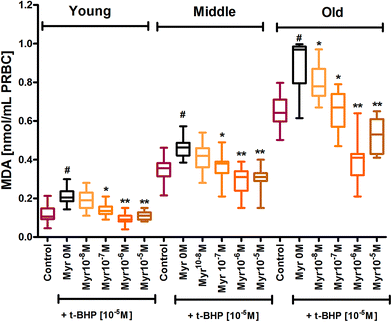
The erythrocyte membrane is prone to lipid peroxidation under oxidative stress that involves cleavage of polyunsaturated fatty acids at their double bonds leading to the formation of MDA. The results of our study showed an age dependent increase in lipid peroxidation in erythrocytes, as evidenced by an increase in MDA content in mixed erythrocyte population during human aging. Subjecting erythrocytes to oxidative stress by incubation with t-BHP caused a significant (p < 0.001) increase in lipid peroxidation in all age groups compared to their respective controls. In vitro administration of quercetin and myricetin provide protection against oxidative stress induced increase in MDA generation in all age groups. Quercetin caused significant (p < 0.01) decrease in MDA content as compared to zero (t-BHP treated only) at 10−8 M and 10−7 M in all 3 age group erythrocytes. The effect was more significant (p < 0.001) at 10−6 M and 10−5 M in all 3 age groups (Fig. 2). Variation was significantly different in old age groups at 10−6 M and 10−5 M quercetin.Myricetin at 10−8 M did not showed any effect in young and middle age group but showed significant (p < 0.01) decrease in MDA content as compared to zero flavonoid. Myricetin at 10−6 and 10−5 M showed more protection as evident by significant decrease in MDA content as compared to respective oxidative injured (zero flavonoid) group (Fig. 3).
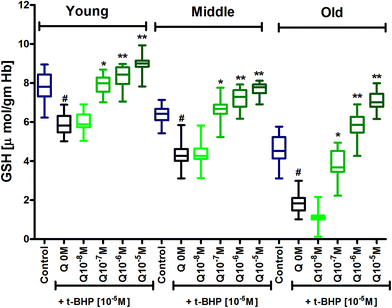
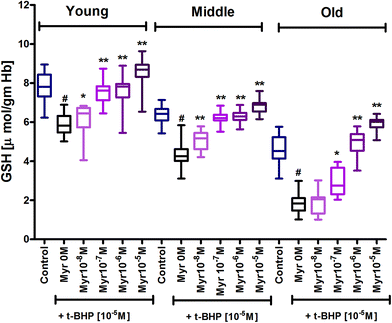
Glutathione (GSH) is a primary antioxidant of red blood cells which provide protection against ROS/RNS. Results showed an age dependent decrease in GSH content in erythrocytes, as demonstrated by a decrease in GSH level in mixed erythrocyte population during human aging. Subjecting erythrocytes to oxidative stress by incubation with t-BHP caused a significant (p < 0.001) decrease in GSH in 3 age groups compared to their respective controls. In vitro treatment of quercetin and myricetin provide protection against oxidative stress induced decrease in GSH level in all age groups. Quercetin caused significant (p < 0.001) increase in GSH level compared to zero quercetin at 10−6 M and 10−5 M in all 3 age group erythrocytes. The effect was also significant (p < 0.01) at 10−7 M but insignificant at 10−8 M in all 3 age groups (Fig. 4). Myricetin at 10−8 M was insignificant in old age group while 10−6 M and 10−5 M showed significant increase (p < 0.001) in all 3 age group erythrocytes (Fig. 5).
The antioxidant capacity decreased during aging process which was analysed by detecting GSH level. To determine the antioxidative capacity of GSH system the absolute concentration of this might be lesser relevance, but the GSH ratio, calculated as GSH/GSSG, seems to be important to study the antioxidant system of red blood cells. Therefore, we calculated this value and correlated with age. We showed a significant (p < 0.0001) negative correlation (r = −0.8334) between GSH/GSSG as a function of human age (Fig. 6).
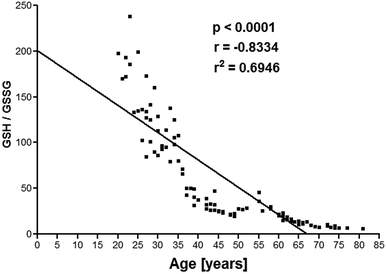 | ||
| Fig. 6 Erythrocyte GSH/GSSG plotted as a function of human age. Concentration of GSSG is expressed in μM g−1 Hb. | ||
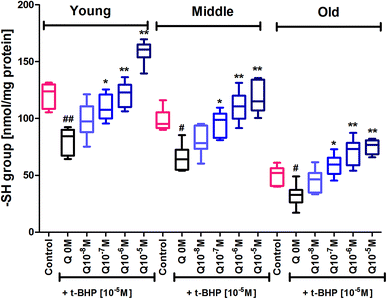
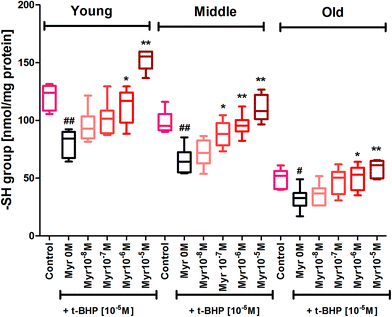
Subjecting erythrocytes to oxidative stress by incubation with t-BHP caused a significant (p < 0.001) decrease in membrane sulfhydryl (–SH) groups in all 3 age groups compared to their respective controls. In vitro treatment of quercetin and myricetin provide significant protection against oxidative stress as evident by increase in –SH level in all age groups. Quercetin caused significant (p < 0.001) increase in –SH level compared to zero quercetin at 10−6 M and 10−5 M in all 3 age group erythrocytes. The effect was also significant (p < 0.01) at 10−7 M but insignificant at 10−8 M in all 3 age groups (Fig. 7). The effect of myricetin was most significant (p < 0.001) at 10−6 M and 10−5 M as compared to zero myricetin. The 10−7 M myricetin showed significant (p < 0.01) effect only in middle age group while 10−8 M myricetin was insignificant in all 3 age group erythrocytes (Fig. 8).
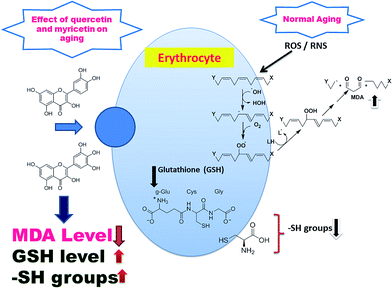
In the current study, we propose that both flavonoids have remarkable antioxidant properties as is evident by significant decreased MDA level while increase in GSH, and membrane –SH groups as compared to injured erythrocytes. The antioxidative effects of these dietary flavonoids is due to a direct scavenging effect on ROS/RNS or an indirect effect on MDA, GSH and membrane –SH groups. Epidemiological studies suggest that dietary flavonoids protect against oxidative stress during human aging.31,32 The beneficial effects of flavonoids have been attributed to their antioxidant properties, including their ability to scavenge O2−˙ and ˙OH and to inhibit lipid peroxidation in a variety of experimental setting. ROS/RNS scavenging efficiency is associated with the presence of hydroxyl groups (OH) in the B-ring of the flavonoids.33 Quercetin and myricetin share the same main structure of hyperoside which provides both the flavonoids with protective properties against oxidative stress.34 Both have ROS/RNS scavenging effects due to formation of less reactive phenoxyl radicals by donating electrons or hydrogen and in between the ability to chelate transition metal ions such as iron. Chelating properties of flavonoids depend on the structural features like the catechol group on the B-ring, 4-carbonyl, and 5-hydroxyl group. Thus, on the basis of structural criteria accounting for the metal chelation, both flavonoids might have possible metal binding sites between the 5-hydroxy and 4-carbonyl group, or between 3′- and 4′-hydroxyl group, thereby chelating metal ions.
Given the antioxidant properties of both flavonoids and the link between aging and oxidative stress,35,36 these flavonoids mediated protection against aging has been studied. We report here that these flavonoids can directly ameliorate t-BHP induced oxidative injury in erythrocytes during aging in humans. Our findings clearly show that quercetin and myricetin have potent antioxidant properties that consequently influence cellular aging, survival, and viability. To the best of our knowledge, with this study, we are the first to present protective effect of quercetin and myricetin in human RBCs by detecting MDA, GSH and –SH group during aging.
MDA, one of the lipid oxidation products, can react with the free amino groups (–NH2) of proteins, phospholipids, and nucleic acids leading to structural modification, which in turn induces dysfunction of immune systems.37 In our earlier studies, we have reported a significant age associated increase in MDA levels.38 A high level of lipid peroxidation products can be detected in cell degradation after cell injury. The rise in the number of lipid oxidation products are found in diabetes, atherosclerosis, liver disease, and inflammation.16 Several findings have emphasized the importance of lipid peroxidation in relation to the role of caloric restriction and the extension of longevity.39 Long lived mammals and birds possess low degrees of unsaturation in their cellular membranes, which leads to lower levels of lipid peroxidation and lipoxidation-derived protein modification in long lived species.40 All the above cited literature supports our finding that the level of MDA is decreased by quercetin and myricetin.
As representative part of the antioxidant cascade, which can be easily measured in blood we selected GSH, with its reduced (GSH) and oxidised (GSSG, glutathione disulfide) form. The GSH and membrane –SH groups represents the main antioxidants that are affected by oxidative stress during aging. GSH is a major intracellular non-protein sulfhydryl compound and is accepted as the most important intracellular hydrophilic antioxidant. Reduced glutathione has many biological functions, including maintenance of membrane protein –SH groups in the reduced form, the oxidation of which can otherwise cause altered cellular structure and function.41 In our results, the GSH/GSSG ratio is age dependent over the age of the individual. This finding may help to explain the shift in the redox status during human aging. The human erythrocyte is an easily accessible cell type that is rich in sulfhydryl functions: the importance of the erythrocyte –SH group in overall cellular redox balance has been emphasized. Membrane oxidative damage has a considerable effect on membrane mechanical properties. Membrane –SH group oxidative damage may be an important molecular mechanism inducing changes in the membrane microelasticity or whole cell deformability under conditions of physiological and pathological oxidative stress.42 The altered redox balance with increasing age may affect the activity of virtually any protein since essentially all proteins contain cysteine and methionine, amino acids which are subject to oxidation–reduction changes. In our previous reports, we had showed a significant decline of the intracellular GSH level and –SH groups during aging.38 It is also worth noting that previous reports had shown increase in GSH level on treatment with these flavonoids which help in neuroprotection.43 Results of the present study show a significant increase in GSH and membrane –SH groups on treatment with quercetin and myricetin, a very important modification associated with aging (Fig. 9).
The current knowledge on the protective effect of both flavonoids on biomarkers of oxidative stress seems to correlate with its capacity to suppress increased oxidative injury and related inflammation stress in erythrocytes. The effect of aging on quercetin and myricetin bioavailability is unclear due to limited studies in this area.44,45
Experimental
Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) following the method of Tietze.49 Standard curve was determined with freshly prepared standard solution of 33 mM GSSG. Concentration of GSH and GSSG were expressed as μmol g−1 Hb. The concentration of –SH group is expressed as nmol per mg protein. Hemoglobin (Hb) was measured using Drabkin's reagent and absorbance measurement at 546 nm.50
20) following the method of Tietze.49 Standard curve was determined with freshly prepared standard solution of 33 mM GSSG. Concentration of GSH and GSSG were expressed as μmol g−1 Hb. The concentration of –SH group is expressed as nmol per mg protein. Hemoglobin (Hb) was measured using Drabkin's reagent and absorbance measurement at 546 nm.50Oxidative stress was induced in vitro by incubating washed erythrocyte and (or) erythrocyte ghosts with 10−5 M tert-butyl hydroperoxide (t-BHP) (final concentration) for 60 min at 37 °C. The concentration and duration of t-BHP used to induce oxidative stress in erythrocytes was the same as described in previously published report.29 The effect of quercetin and myricetin was evaluated by co-incubating erythrocytes in the presence of quercetin and myricetin (10−8 M to 10−5 M) (final concentration) and t-BHP (10−5 M) for 60 min at 37 °C with mild shaking. We selected flavonoid concentrations in the present study following a previous report in human RBCs.18 Erythrocytes were again washed two to three times with KRP, pH 7.4 and finally; packed red blood cells (PRBCs) were used for the assay. In parallel, for control experiments, blood was incubated without flavonoids. For membrane –SH group content estimation, erythrocyte ghosts (0.4–0.6 mg protein) were incubated with indicated final concentrations of quercetin and myricetin in 3 mL of 0.1 mol L−1 phosphate buffer for 60 min at 37 °C before estimation of membrane –SH groups.
Statistical analyses
All statistical analyses were performed using the statistical Package for Social Science (SPSS, version 20.0 for Windows). The effect of quercetin and myricetin were analysed using multivariate ANOVA (MANOVA). Post hoc testing for within group comparison (control, t-BHP treated (zero flavonoid), t-BHP + flavonoids) for young, middle, and old. Relationship between GSH/GSSG and age were assessed using Pearson correlation coefficient (r) and coefficient of determination (r2). A probability (p) value of less than 0.05 was considered statistically significant.Conclusions
Many attempts are made towards healthy aging and longevity. The direct detection of MDA, GSH, and –SH in quercetin and myricetin treated RBCs will provide helpful information in anti-aging research. The capability of quercetin and myricetin to act as antioxidant to increase the GSH and membrane –SH level while decreased MDA level during aging definitely represents a fascinating tool in the field of anti-aging research. It is anticipated that the use of these flavonoids in targeting ROS metabolism could bypass drug resistance and achieve selectivity of treatment in various pathological conditions. Despite of this it can also be suggested that these flavonoids can be used as an alternative for available therapeutic strategies for aging and age related disorders. Further research work should be directed towards finding the molecular mechanism underlying these effects. Thus, age related changes by both flavonoid and their bioavailabilities require more comprehensive investigation through detection of other available age related biomarkers. Finally, we conclude that the beneficial role of quercetin and myricetin during aging in humans is connected to its antioxidant action. These findings emphasize the need to establish age-dependent reference values of dietary flavonoids for oxidative stress biomarkers in different populations, and in studies involving their role in different disease conditions.Acknowledgements
PKM and PK acknowledge Amity University Uttar Pradesh, Noida for providing necessary research facility and PKM also acknowledge support by fellowship (Science without Borders-Level A) from coordination of Improvement of Higher Education Personnel (CAPES), National Counsel of Technological and Scientific Development (CNPq), Brazil. PC acknowledges IIT Guwahati for providing necessary facility for completion of this work.Notes and references
- D. Harman, Biogerontology, 2009, 10, 773–781 CrossRef CAS PubMed.
- G. Lopez-Lluch, C. Santos-Ocana, J. A. Sanchez-Alcazar, D. J. Fernandez-Ayala, C. Asencio-Salcedo, J. C. Rodriguez-Aguilera and P. Navas, Biogerontology, 2015, 16(5), 599–620 CrossRef CAS PubMed.
- N. Kumar, P. K. Maurya, R. Kant and S. I. Rizvi, Arch. Physiol. Biochem., 2016, 122, 155–160 CrossRef CAS PubMed.
- D. P. Jones, Redox Biol., 2015, 5, 71–79 CrossRef CAS PubMed.
- J. M. Gutteridge and B. Halliwell, Ann. N. Y. Acad. Sci., 2000, 899, 136–147 CrossRef CAS PubMed.
- Z. Nikitaki, C. E. Hellweg, A. G. Georgakilas and J. L. Ravanat, Front. Chem., 2015, 3, 35 Search PubMed.
- P. K. Maurya, P. Kumar, N. Siddiqui, P. Tripathi and S. I. Rizvi, Indian J. Biochem. Biophys., 2010, 47, 319–321 CAS.
- P. K. Maurya and S. I. Rizvi, Indian J. Clin. Biochem., 2010, 25, 398–400 CrossRef CAS PubMed.
- S. I. Rizvi and P. K. Maurya, Mol. Biotechnol., 2007, 37, 58–61 CrossRef CAS PubMed.
- M. Gonzalez-Freire, R. de Cabo, M. Bernier, S. J. Sollott, E. Fabbri, P. Navas and L. Ferrucci, J. Gerontol., Ser. A, 2015, 70(11), 1334–1342 CrossRef PubMed.
- S. I. Rizvi and P. K. Maurya, Rejuvenation Res., 2008, 11, 661–665 CrossRef CAS PubMed.
- D. P. Jones, V. C. Mody Jr, J. L. Carlson, M. J. Lynn and P. Sternberg Jr, Free Radical Biol. Med., 2002, 33, 1290–1300 CrossRef CAS PubMed.
- K. B. Pandey and S. I. Rizvi, Oxid. Med. Cell. Longevity, 2010, 3, 2–12 CrossRef PubMed.
- L. Gil, W. Siems, B. Mazurek, J. Gross, P. Schroeder, P. Voss and T. Grune, Free Radical Res., 2006, 40, 495–505 CrossRef CAS PubMed.
- P. K. Maurya, P. Kumar and P. Chandra, World J. Meth., 2015, 5, 216–222 Search PubMed.
- Y. Yoshida, A. Umeno, Y. Akazawa, M. Shichiri, K. Murotomi and M. Horie, J. Oleo Sci., 2015, 64, 347–356 CrossRef CAS PubMed.
- H. B. Noh, P. Chandra, J. O. Moon and Y. B. Shim, Biomaterials, 2012, 33, 2600–2607 CrossRef CAS PubMed.
- P. Kumar, S. Chand and P. K. Maurya, Arch. Physiol. Biochem., 2016, 1–26 Search PubMed.
- A. Costa, M. Y. Bonner and J. L. Arbiser, Am. J. Clin. Dermatol., 2016, 1–17 Search PubMed.
- C. Alasalvar and B. W. Bolling, Br. J. Nutr., 2015, 113(2), S68–S78 CrossRef CAS PubMed.
- N. Terahara, Nat. Prod. Commun., 2015, 10, 521–528 Search PubMed.
- A. Aras, A. R. Khokhar, M. Z. Qureshi, M. F. Silva, A. Sobczak-Kupiec, E. A. Pineda, A. A. Hechenleitner and A. A. Farooqi, Asian Pacific Journal of Cancer Prevention: APJCP, 2014, 15, 3865–3871 CrossRef PubMed.
- R. Domitrovic, K. Rashed, O. Cvijanovic, S. Vladimir-Knezevic, M. Skoda and A. Visnic, Chem.–Biol. Interact., 2015, 230, 21–29 CrossRef CAS PubMed.
- K. Valentova, P. Sima, Z. Rybkova, J. Krizan, K. Malachova and V. Kren, J. Sci. Food Agric., 2015, 96(5), 1492–1499 CrossRef PubMed.
- N. Hayder, I. Bouhlel, I. Skandrani, M. Kadri, R. Steiman, P. Guiraud, A. M. Mariotte, K. Ghedira, M. G. Dijoux-Franca and L. Chekir-Ghedira, Toxicol. In Vitro, 2008, 22, 567–581 CrossRef CAS PubMed.
- Y. Guo and R. S. Bruno, J. Nutr. Biochem., 2015, 26, 201–210 CrossRef CAS PubMed.
- P. Kumar and P. K. Maurya, Adv. Pharm. Bull., 2014, 4, 443–447 CAS.
- P. K. Maurya and S. Prakash, Phytother. Res., 2011, 25, 944–946 CrossRef CAS PubMed.
- P. K. Maurya and S. I. Rizvi, Nat. Prod. Res., 2009, 23, 1072–1079 CrossRef CAS PubMed.
- F. Dai, Q. Miao, B. Zhou, L. Yang and Z. L. Liu, Life Sci., 2006, 78, 2488–2493 CrossRef CAS PubMed.
- C. Peng, X. Wang, J. Chen, R. Jiao, L. Wang, Y. M. Li, Y. Zuo, Y. Liu, L. Lei, K. Y. Ma, Y. Huang and Z. Y. Chen, BioMed Res. Int., 2014, 2014, 831841 Search PubMed.
- M. H. Pan, C. S. Lai, M. L. Tsai, J. C. Wu and C. T. Ho, Mol. Nutr. Food Res., 2012, 56, 88–115 CAS.
- N. Cotelle, J. L. Bernier, J. P. Catteau, J. Pommery, J. C. Wallet and E. M. Gaydou, Free Radical Biol. Med., 1996, 20, 35–43 CrossRef CAS PubMed.
- C. Rice-Evans, Curr. Med. Chem., 2001, 8, 797–807 CrossRef CAS PubMed.
- N. Chondrogianni, S. Kapeta, I. Chinou, K. Vassilatou, I. Papassideri and E. S. Gonos, Exp. Gerontol., 2010, 45, 763–771 CrossRef CAS PubMed.
- S. J. Duthie, A. R. Collins, G. G. Duthie and V. L. Dobson, Mutat. Res., 1997, 393, 223–231 CAS.
- O. M. Panasenko, T. V. Vol'nova, O. A. Azizova and Y. A. Vladimirov, Free Radical Biol. Med., 1991, 10, 137–148 CrossRef CAS PubMed.
- S. I. Rizvi and P. K. Maurya, Ann. N. Y. Acad. Sci., 2007, 1100, 373–382 CrossRef CAS PubMed.
- B. P. Patel, A. Safdar, S. Raha, M. A. Tarnopolsky and M. J. Hamadeh, PLoS One, 2010, 5, e9386 Search PubMed.
- R. Pamplona, M. Portero-Otin, A. Sanz, V. Ayala, E. Vasileva and G. Barja, Age, 2005, 27, 267–280 CrossRef CAS PubMed.
- S. Szabo, L. Nagy and M. Plebani, Clin. Chim. Acta, 1992, 206, 95–105 CrossRef CAS.
- O. Gorelenkova Miller and J. J. Mieyal, Arch. Toxicol., 2015, 90(4), 1019–1020 CrossRef PubMed.
- S. Chaudhary, P. Ganjoo, S. Raiusddin and S. Parvez, Protoplasma, 2015, 252, 209–217 CrossRef CAS PubMed.
- F. Jin, D. C. Nieman, R. A. Shanely, A. M. Knab, M. D. Austin and W. Sha, Eur. J. Clin. Nutr., 2010, 64, 692–697 CrossRef CAS PubMed.
- B. W. Bolling, M. H. Court, J. B. Blumberg and C. Y. Chen, J. Nutr. Biochem., 2010, 21, 498–503 CrossRef CAS PubMed.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- H. Esterbauer and K. H. Cheeseman, Methods Enzymol., 1990, 186, 407–421 CAS.
- H. Kitajima, T. Yamaguchi and E. Kimoto, J. Biochem., 1990, 108, 1057–1062 CAS.
- F. Tietze, Anal. Biochem., 1969, 27, 502–522 CrossRef CAS PubMed.
- Br. J. Haematol., 1967, 13, 71–75 Search PubMed.
Footnote |
| † These authors contributed equally to the study. |
| This journal is © The Royal Society of Chemistry 2016 |