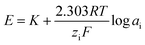Research progress in electroanalytical techniques for determination of antimalarial drugs in pharmaceutical and biological samples
Neeta Thapliyal*a,
Tirivashe E. Chiwunzea,
Rajshekhar Karpoormath*a,
Rajendra N. Goyalb,
Harun Patela and
Srinivasulu Cherukupallia
aDepartment of Pharmaceutical Chemistry, College of Health Sciences, University of KwaZulu-Natal, Durban 4000, South Africa. E-mail: karpoormath@ukzn.ac.za; thapliyaln@ukzn.ac.za; Fax: +27-312607792; Tel: +27-312607179
bDepartment of Chemistry, Indian Institute of Technology Roorkee, Roorkee 247667, India
First published on 10th June 2016
Abstract
Antimalarial drugs play a crucial role in the treatment and cure of malaria. Electrochemical sensors have been the subject of extensive research in the field of drug analysis since many decades, and yet seem to project great potential for the future. The present review explores the basic strategies and recent developments in the electroanalysis of antimalarial drugs in various matrices. A discussion of the commonly used electroanalytical methods for drug assays is briefly discussed. The methods have been critically analysed highlighting their analytical performance and limitations. Nanomaterials, conducting polymers and presence of surfactants made significant contributions to voltammetric determination of antimalarial drugs suggesting promising future for use of chemically-modified electrode based sensors for the detection. Future progress in quantification of these drugs in biological fluids is expected to drastically improve the applicability of electrochemical methods for routine clinical analysis.
1. Introduction
Malaria, a mosquito-borne infectious disease, continues to be one of the most severe health threats worldwide with an estimated 198 million cases of the disease in 2013, resulting in 584![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths per year.1 Almost half of the world's population is at risk of contracting malaria with most of the related deaths reported among children residing in sub-Saharan Africa, where a child succumbs to the disease every minute. Malaria is caused by parasitic protozoans of the genus Plasmodium with its five species (Plasmodium vivax, Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale and Plasmodium knowlesi) affecting the human race, the most deadly being P. falciparum.2 Prompt diagnosis and treatment of the disease remarkably lowers the associated morbidity and mortality rate. Antimalarial drugs play a vital role in reducing malaria transmission and restricting the spread of drug-resistant infecting species. Currently, there are four major drug classes used for the management of the disease, which include quinoline-related compounds, artemisinin derivatives, antifolates and antimicrobials. No single drug available is effective against all forms of the parasite's life cycle and hence a combination of drugs is recommended to combat the disease. Despite the availability of a wide arsenal of antimalarials, continuous efforts are in progress to develop and assay new drugs in order to improve the efficacy of the therapy. Resistance to antimalarial medicines pose a problem for effective malarial therapy;3 however use of drugs still remain the most effective option for malaria treatment.
000 deaths per year.1 Almost half of the world's population is at risk of contracting malaria with most of the related deaths reported among children residing in sub-Saharan Africa, where a child succumbs to the disease every minute. Malaria is caused by parasitic protozoans of the genus Plasmodium with its five species (Plasmodium vivax, Plasmodium falciparum, Plasmodium malariae, Plasmodium ovale and Plasmodium knowlesi) affecting the human race, the most deadly being P. falciparum.2 Prompt diagnosis and treatment of the disease remarkably lowers the associated morbidity and mortality rate. Antimalarial drugs play a vital role in reducing malaria transmission and restricting the spread of drug-resistant infecting species. Currently, there are four major drug classes used for the management of the disease, which include quinoline-related compounds, artemisinin derivatives, antifolates and antimicrobials. No single drug available is effective against all forms of the parasite's life cycle and hence a combination of drugs is recommended to combat the disease. Despite the availability of a wide arsenal of antimalarials, continuous efforts are in progress to develop and assay new drugs in order to improve the efficacy of the therapy. Resistance to antimalarial medicines pose a problem for effective malarial therapy;3 however use of drugs still remain the most effective option for malaria treatment.
Development of new drugs with varying pharmacological action and therapeutic properties presents a challenging task to control the content of drugs as well as their metabolites in different media, including commercial formulations and biological fluids. Therapeutic drug monitoring (TDM) is a clinical strategy that measures drug concentration in a patient's bloodstream to monitor its compliance, efficacy and toxicity, thus optimizing and managing individual medication regimen. TDM also investigates occurrence of adverse effects and drug–drug interactions. Hence robust, precise and accurate analytical methods are required to quantify the drugs in human body fluids. Besides, since therapeutic substances and preparations need to be free from any impurities and also to detect counterfeiting of medicines, quantitative analysis of drugs in commercial formulations is critical for effective quality control. Based on these facts it becomes imperative to emphasize on the development of analytical methodologies for quantification of antimalarial drugs in pharmaceutical as well as biological samples. A large number of methods have been reported for the assay of antimalarial drugs, most of which are time-consuming and involve labour-intensive, costly and highly sophisticated chromatographic techniques.4–13 However, last few decades witnessed an upsurge in electrochemical methods for the detection and determination of these compounds. The electrochemical techniques are characterized by high selectivity and sensitivity, minimum or no sample preparation and extremely low detection limit, which is comparable to widely used chromatographic techniques. In addition, low cost instrumentation, portability and versatility for the analysis of a wide variety of species favours electrochemistry technique as the analytical method of choice.1 Electrochemical analysis is now increasingly used for drug analysis in biological samples and their dosage forms. This work presents a critical review of the electrochemical methods reported in the literature for the determination of WHO recommended antimalarial drugs, namely chloroquine, quinine, mefloquine, piperaquine, primaquine, amodiaquine, lumefantrine, artemisinin, artemether, dihydroartemisinin, artesunate, atovaquone, proguanil, sulfadoxine, pyrimethamine, doxycycline and clindamycin. These methods constitute useful tools having practical applicability for quality control tests and/or routine TDM in clinical practice. The review is an attempt to critically discuss the electrochemical methods developed till date for quantitative analysis of the above-mentioned drugs revealing their advantages and limitations and throw light on the recent trends in antimalarial drug determination.
2. Brief account of most frequently used electroanalytical techniques
Electroanalytical techniques are powerful, versatile and simple techniques of analysis that offer wide linear concentration range, rapid analysis time, cost-effective instrumentation, possibility of miniaturization, low reagent consumption, suitability for real-time detection along with high sensitivity, precision and appreciable accuracy.14–16 Among the various known electrochemical methods, voltammetry and potentiometry are the most prominent ones widely used for drug quantification.2.1 Voltammetry
Voltammetry involves measurement of the current (i) generated upon application of a constant or varying potential (E) at the working electrode surface. Since the measured current is directly proportional to the bulk concentration of the analyte species, the method allows quantitative analysis of the analyte with extreme sensitivity. In some cases, the current is recorded or the applied potential is varied over a period of time (t). When the current is recorded at a constant potential, the technique is termed amperometry. Thus, all voltammetric techniques can be defined as some function of current, potential and time. Since the applied potential electrochemically oxidizes or reduces the analyte species resulting in a change in its concentration at the electrode surface, voltammetry is considered as an active technique. The most widely used working electrodes are carbon, gold, silver, platinum and mercury. The advent of chemically modified electrodes have increased the scope of electrochemical sensing by improving electrode sensitivity allowing detection of a wide range of electroactive compounds upto a very low detection limit.17 The technique is extensively employed for trace analysis of various inorganic and organic electrochemical species in different matrices (for example, clinical samples, pharmaceutical formulations, environmental samples, gasoline and oil).18–20 Numerous voltammetric techniques in electroanalytical chemistry, namely, cyclic, linear sweep, differential, square wave and stripping voltammetry, have been developed in recent decades. A brief overview of the basic concepts of the various commonly employed electroanalytical techniques is summarized below.| id = 706nCD1/2m2/3t1/6 |
Qualitative information can also be obtained from the half-wave potential of the polarograms. Though mercury displays advantages as a working electrode such as wide cathodic range and a renewable surface, it has several limitations that include its non-suitability for investigating oxidizable species. Besides, potential risks of contamination, poisoning and disposal associated with the use of mercury have also emerged as a big problem.
| ip = [2.69 × 105]n3/2AD1/2Cv1/2 |
2.1.4.1 Differential pulse voltammetry. Differential pulse voltammetry (DPV) is a prominent voltammetric technique extensively employed for quantitative analysis of electrochemically active species. It provides superior detection limits in comparison to LSV and CV, and also allows resolution of overlapping electrochemical processes. In this technique, the potential applied to the electrode is varied in a series of steps (or pulse) from an initial potential to an interlevel potential where it remains for approximately 50 milliseconds and then changes to the final potential. In the recurring pulses, the final potential is changed, and a constant difference is maintained between the initial and the interlevel potential. The current is sampled twice, first just before application of each pulse and second time at the end of the pulse. The difference between these values is plotted as a function of the potential. Because of low limit of detection (∼10−8 M), DPV is often the method of choice for analysis of pharmaceutical formulations and body fluids.
2.1.4.2 Square-wave voltammetry. Square-wave voltammetry (SWV) is one of the most powerful pulse techniques demonstrating a broader dynamic range and lower limit of detection. The potential wave form in SWV consists of a symmetrical square wave superimposed on a staircase (stepped) potential ramp in such a way that the forward square wave pulse coincides with the underlying staircase. The current is measured twice during each square-wave cycle, once at the end of the forward pulse, and then at the end of the reverse pulse. The difference between the two measurements is plotted versus the applied staircase potential. The resulting peak-shaped voltammogram displays excellent sensitivity and effective discrimination against background contributions. SWV is considered more sensitive than DPV because in it both forward and reverse currents are measured. Speed, excellent sensitivity and the rejection of background currents are some of the advantages of using SWV. Thus, the analysis time is reduced and analytical determinations can be made at very low concentrations. Applications of SWV include trace analysis of electrochemical species and its use in electrochemical detection with HPLC.
In ASV, the analyte is deposited (or electroplated) onto the working electrode at negative potentials and then oxidized during an anodic potential sweep resulting in a sharp peak. During deposition, an amalgam is formed by the elemental metal and the mercury on the electrode. ASV can only be used to determine those metals that exhibit appreciable solubility in mercury. CSV is used to determine substances that form insoluble salts with mercurous ion. In this method, a positive potential is applied to the mercury electrode in the presence of such a substance resulting in the formation of an insoluble film on the electrode surface. Application of a potential scan in the negative direction reduces (or strips) the deposited film into the solution. The method is employed to detect inorganic anions and certain organic compounds. AdSV is similar to ASV and CSV except that here the preconcentration step is achieved simply by adsorption on the working electrode surface or by reactions at chemically modified electrodes. Many organic and inorganic species have been determined at micromolar and nanomolar concentration levels using this technique.
The analytical advantages of the various voltammetric techniques include excellent sensitivity with a very wide linear concentration range for both organic and inorganic species (10−12 to 10−1 M), rapid and easy analysis, simultaneous determination of several analytes, the ability to determine kinetic and mechanistic parameters, having a well-developed theory and thus the ability to rationally estimate the values of unknown parameters. However, this method is applicable to limited range of metals, and organic compounds present in the sample need to be completely destroyed prior to determining the inorganic species in solution.
2.2 Potentiometry
Potentiometry relates to measurement of the potential between two electrodes in an electrochemical system providing information about the quantity of the analyte species. These methods measure potential as a function of analyte activity or concentration and find use in a wide range of applications such as industrial quality control, environment pollution monitoring, food processing, agriculture and biomedical analysis.21–23 There are two broad classes of potentiometric electrodes, namely the metallic electrodes and membrane electrodes. The potential of a metallic electrode is the result of a redox reaction at the electrode's surface. An electrode of the first kind responds to the concentration of its cation in solution. If another species is in equilibrium with the metal ion, the electrode's potential also responds to the concentration of that species. The potential of a membrane electrode is determined by a difference in the concentration of the solution on each side of the membrane. The recent potentiometric methods mostly use ion-selective electrodes (ISEs) that are capable of selectively binding the analyte ions attaining lower detection limits. ISEs are highly sensitive permselective membrane-based devices consisting of permselective ion-conducting materials. The membrane is generally water insoluble, mechanically stable, nonporous and is designed such that it will selectively bind the analyte ions. The key component of the membranes is a complexing agent capable of reversible binding with the target ion. It is generally called ionophore or electroactive material. Besides ionophore, other components of the membranes include solvent mediators or plasticizers and anionic or cationic additives which also affect the potentiometric characteristics of ISEs. A potential is developed across the membrane when it separates two solutions of different activity containing the same counter ion. The monitored cell potential is found to be directly proportional to the concentration or activity of the sample ions in aqueous solution under investigation. Ideally, the response of ISE should follow the given equationwhere E is the potential, K is a constant that includes all sample-independent potential contributions, R is the universal gas constant, F is the Faraday constant, T is the absolute temperature, and zi and ai are the ionic charge and activity of the ion, respectively. At 25 °C, the value of 2.303RT/ziF is 0.059/zi volts. The membrane is said to exhibit Nernstian response if the slope of a plot between cell potential and log activity comes out to be 0.059/zi volts. The plot is then called Nernst plot and slope as Nernstian slope. ISEs provide rapid, accurate, low cost and on-line method of analysis. Limitations of the method include its dependency on temperature. Also, the ISE is usually fragile and can be fouled by solution components leading to sluggish and drifting response. Potentiometry with ISEs is one of the most promising analytical tools for determining various organic and inorganic species which has led to a constant increase in development of electrodes endowed with the ability to selectively identify a number of drugs for pharmaceutical and clinical analyses.
A comparison of voltammetric and potentiometric methods of analysis is presented in Table 1. Both methods have their respective advantages and disadvantages, along with different scopes of analytes and matrix effects, making them appropriate or advantageous for analysis of a specific analyte. Also, both techniques are well-suited for in situ continuous monitoring and are accessible for miniaturization, integration and automation.
| Technique | Voltammetry | Potentiometry |
|---|---|---|
| Type | Dynamic technique since it involves change in analyte concentration | Passive technique since it does not affect the concentration of the analyte in the sample |
| Method of operation | Measures oxidation or reduction current as a function of voltage | Measures potential at zero current |
| Analyte | Should either be electroactive or can be measured by an electroactive species in an indirect manner | No such limitation |
| Measurement | Measures concentration | Measures activity rather than concentration |
| Detection limits | ∼10−9 M or even lower (ca. 10−12 M) | ∼10−6 M but can be lower (ca. 10−8 M) |
| Principal applications | Quantitative analysis of electrochemically reducible organic or inorganic substances | Quantitative analysis of ions in solutions, pH |
3. Electrochemical methods for quantification of antimalarial drugs
Antimalarials have been categorised into different classes that include the quinoline derivatives, artemisinin derivatives, antifolates and antimicrobials. The essential pharmacokinetic properties of the antimalarials are summarized in Table 2. These antimalarials are well absorbed after oral administration and show high bioavailability. Each analytical technique is known to have its own characteristics that vary from one analyte to another. Method validation is a pre-requisite step to assess the performance characteristics of analytical methods. It provides reliable analytical data with the basic parameters being linearity, concentration range, limit of detection (LOD) and limit of quantification (LOQ). Hence, it is essential to consider the validation parameters for effective analysis of the various methods used to determine the drug. Keeping this in view, the validation characteristics reported for the electroanalytical methodologies discussed have been highlighted in the present review.| Antimalarial drug | Cmax (ng mL−1) | Tmax (hours) | UE (%) | Adverse effects/overdose | Ref. |
|---|---|---|---|---|---|
| Chloroquine | 63–263 | 1–8 | 39–67 | ECG disturbance, leucopenia, methaemoglobinaemia, toxic psychosis peripheral neuropathy, retinopathy, keratopathy, myopathy, abdominal cramps, vomiting, diarrhoea, pruritus, alopecia, exfoliative dermatitis, purpuric skin reactions and hyperpigmentation | 24–26 |
| Quinine | 5270–17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.0–5.9 | 20 | Cinchonism, visual impairment, hypoglycaemia, tinnitus, hearing impairment, headache, diplopia, rash, dermatitis, vomiting, stomach pain, diarrhea, central nervous system toxicity, pulmonary edema, cardiac arrhythmia and death (in rare cases) | 27 and 28 |
| Mefloquine | 722–2259 | 4.5–31 | 9 | Depression, anxiety, hallucinations, severe or stomach pain, nausea, vomiting, loss of appetite, dark urine, clay-colored stools, jaundice, hepatitis, mouth sores, weight loss, severe skin rash, polyneuropathy, bradycardia, visual impairment, thrombocytopenia, psychosis, seizures, acute brain syndrome | 28 and 29 |
| Amodiaquine | 5.2–39.3 | 0.5–2.0 | 2.0 | Gastrointestinal disturbances, cough, anorexia, insomnia, fatigue, neutropenia, hepatotoxicity, arrhythmia, bradycardia, pruritus, eye disorders. Bone marrow toxicity and hepatotoxicity | 28 and 30 |
| Primaquine | 65–295 | 1.0–4.0 | 0.4–2.2 | Gastrointestinal disorders, hypertension, cardiac arrhythmia, pruritus, headache, confusion, depression, haemolysis, leukopenia, granulocytopenia, methemoglobinemia (cyanosis) | 28 and 31 |
| Piperaquine | 71.6–730 | 3.0–6.0 | <1% | Dizziness, headache, nausea, vomiting, anorexia, myalgia, cough, asthenia, arthralgia, abdominal distress, pyrexia, eosinophilia, QTc prolongation | 28 and 32 |
| Lumefantrine | 5100–9800 | 6–8 | Traces only | Headache, dizziness, loss of appetite, weakness, fever, chills, muscle/joint pain, nausea, cough, abdominal and chest pain, irregular heartbeat | 28, 33 and 34 |
| Artemisinin | 211–1056 | 1–5 | <1.0 | Nausea, vomiting, abdominal pain, diarrhoea, abdominal pain, fever, darkening of urine, dizziness, hypertension, skin rashes, sweating, tinnitus, cardiovascular disturbances, hepatitis | 28 and 35 |
| Artesunate | 47.5–223.2 | 0.5–1.5 | Traces only | Transient and reversible reticulocytopenia, fever, rash, ataxia, bradycardia, transient first-degree heart block and reversible elevation of serum transaminases | 28 and 36 |
| Artemether | 287–648 | 1.5–2.5 | Traces only | Hypersensitivity reactions, gastrointestinal disturbances, bradycardia, neutropenia, reticulocytopenia and elevated liver enzyme activity | 28, 37 and 38 |
| Dihydroartemisinin | 162–702 | 2–12 | 52 | Headache, nausea, loss of appetite, dizziness, energy loss, muscle/joint pain, rash, coloured urine, jaundice, arthralgia, abdominal distress, neutropenia, pyrexia | 28 and 37 |
| Proguanil | 560–751 | 4–6 | 30–69 | Rash, abdominal pain, bloody urine, lower back pain, vomiting, diarrhoea, headache, loss of appetite, mouth sores or ulcers, nausea | 28 and 39 |
| Pyrimethamine | 676–1190 | 1–4 | 20–40 | Gastric distress, nausea, vomiting, loss of appetite, bloody urine, chest pain, irregular heartbeat, atrophic glossitis, blood dyscrasias, leucopenia, thrombocytopenia | 28, 38 and 40 |
| Sulfadoxine | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900–217 900–217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4–63 | 3.0 | Gastrointestinal disturbances, headache, rash, dizziness, skin reactions, hepatitis, leucopenia, thrombocytopenia, anaemia, haematuria | 28 and 36 |
| Clindamycin | 2.5–14 | 0.75–3.0 | 10 | Diarrhoea, anorexia, nausea, vomiting, abdominal discomfort, pruritus, anaphylaxis, blood dyscrasia, hepatotoxicity, polyarthritis | 28 and 41 |
| Doxycycline | 3060–6900 | 1.5–6.0 | 35–60 | Gastrointestinal effects, hypersensitivity reactions, thrombophlebitis, hypoplasia, enterocolitis and inflammatory lesions in the ano-genital region, candidal vaginitis, rash, dermatitis | 28 and 42 |
| Atovaquone | 634–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 270 270 |
5–6 | <0.6 | Vomiting, diarrhoea, abdominal pain, headache, cough, rash, fever | 28 and 43 |
3.1 Quinoline derivatives
The antimalarial quinoline derivatives comprise of chloroquine, quinine, mefloquine, amodiaquine, primaquine, piperaquine and lumefantrine. The mechanism of action of quinoline antimalarials is not fully known; however these drugs are said to accumulate inside the acidic food vacuole by virtue of their weak base properties, where they interfere with haemoglobin digestion and inhibit the process of heme polymerization, thus facilitating an aggregation of cytotoxic heme eventually killing the parasite. The chemical structures of quinoline based antimalarial drugs are shown in Fig. 1.The voltammetric behaviour of CQ was investigated at bare and dsDNA-modified carbon paste electrode (CPE) in acetate, Britton–Robinson (BR) and phosphate buffer solutions using CV and DPV.46 Presence of dsDNA on the electrode surface facilitated a preconcentration process for CQ resulting in high sensitivity and an improved electrochemical signal. The oxidation peaks observed were assigned to be due to the irreversible oxidation of the nitrogen of alkylamino group and N-heterocyclic nitrogen of the aminoquinoline moiety of CQ molecule. The method was validated for analysis of the drug in serum without any sample pre-treatment with satisfactory recovery range. However, the determination of CQ content in pharmaceutical samples was not conducted. Also, the study did not report the effect of potential interferents as well as the relative affinity of various metabolites towards DNA which may adversely affect the quantification of the drug in biological fluids. Later, in 2009, a Cu(OH)2 nanowire-modified CPE (Cu-NW/CPE) was developed for the electroanalytical determination of CQ in phosphate buffer (pH 5.5).47 The electrode was remarkably stable and yielded reproducible results. Though the method was successfully applied for the determination of CQ in pharmaceutical samples, it was not validated for quantification of the drug in human body fluids.
Various polyvinyl chloride (PVC) based CQ-selective electrodes were developed and evaluated for the assay of CQ in tablets.48–50 The electrodes exhibited Nernstian behaviour and fast response times within a wide pH range. The lifetime of these sensors was found to be relatively short which was anticipated to be due to low lipophilic character of the sensing material having sodium tetraphenylborate (NaTPB) as the membrane additive. Later, an improved CQ potentiometric sensor was proposed that used a more lipophilic ion-exchanger, potassium tetrakis(4-chlorophenyl)borate (KTCPB).51 The electrode showed fast Nernstian response and superior lifetime with minor interference from alkali and alkaline earth metal ions. The developed sensor was then utilised for determination of the drug in synthetic tablets, artificial serum and biological fluids. Unsatisfactory results were obtained for biological fluids and serum samples which indicated the non-usability of the sensor in clinical samples. Another drawback of the method was that the membrane surface swelled and cracked upon exposure to proteins and urine for more than 8 hours.
Qn was determined in tonic waters (containing 23.5–118 mg Qn L−1) in BR buffer (pH 3.7) using d. c. polarography at a dropping mercury electrode (DME).53 A multichannel taste sensor based on CPE modified with lutetium/praseodymium phthalocyanine complexes (LuPc2/PrPc2) or doped polypyrrole (Ppy) was constructed and evaluated to detect bitterness in beverages and food items caused by Qn, MgCl2 and some phenolic compounds.54 The doping agents used were potassium perchlorate, potassium ferricyanide(II), p-toluenesulfonic acid, lithium trifluoromethane sulphonate, potassium hexafluorophosphate, sodium 1-decanesulfonate and sodium tetrasulphonate-phthalocyaninate of nickel(II). LuPc2 modified electrode exhibited appreciable electrocatalytic activity in contrast to PrPc2 electrode, which does not give any response to Qn. Poorly defined peaks were observed at doped Ppy electrodes. The principal component analysis of the obtained signals confirmed the capability of the electrodes for discrimination towards the bitter solutions.54
The electrochemical oxidation of the drug was explored at a multiwall carbon nanotubes (MWCNTs) and room-temperature ionic liquid of 1-butyl-3-methylimidazolium hexafluorophosphate gel modified GCE (MWCNTs–RTIL/GCE) in 0.10 M phosphate buffer solution using CV and SWV.55 No interference was observed from substances commonly present in pharmaceutical preparations (K+, Na+, Cl−, NO3−, SO42−, glucose, saccharose and citrate) suggesting that the method was highly selective towards Qn. The feasibility of the proposed method for quantitative determination of Qn in pharmaceutical injection samples was successfully evaluated. Later, Awasthi et al. studied the cyclic voltammetric behaviour of the drug at a polypyrrole-pentacyanoferrate/platinum (Ppy-PCNFe/Pt) electrode in aqueous medium.56 The work was first of its kind for determination of the drug in absence of any supporting electrolyte. The electrochemical process was irreversible and showed diffusion as well as adsorption controlled behaviour at the electrode surface. However, a major drawback of the method was that a fresh electrode needed to be used for each new measurement since movements of ions into and out of the polymeric film was expected to change the composition of modified electrode. Further, at drug concentrations lower than 1.0 mM, a loss in electrochemical activity of Ppy-PCNFe/Pt electrode was observed due to deactivation of Ppy. No attempts were made to evaluate the analytical applicability of the method for analysis of Qn in any of the real samples. Poly(4-amino-3-hydroxynaphthalene sulfonic acid)-modified glassy carbon electrode (p-(AHNSA)/GCE) was fabricated by electropolymerization and used to determine Qn in 0.1 M phosphate buffer solution using SWV.57 The detection limit obtained was found to be much lower as compared to the earlier reported methods for the quantification of the drug. The influence of potential interferents on the peak current response of the drug revealed significant interference from equimolar quinidine and 8-hydroxyquinoline. The analytical applicability of the method for Qn analysis was successfully validated in pharmaceutical preparations (tablets and injections) and spiked human urine samples. However, the stability, accuracy and reproducibility of the method were not mentioned. A molecularly imprinted polymer (MIP) film with methacrylic acid (MAA) as functional monomer and ethylene glycol maleic rosinate acrylate (EGMRA) as cross-linker was created on GCE surface via free radical polymerization method.58 The prepared sensor showed sensitive and selective binding sites for Qn and was employed for Qn determination. Though cinchonine, cinchonidine, caffeine and theophylline did not affect the amperometric response towards the drug, a significant interference was observed in presence of tenfold of quinidine. The method was found to be accurate, reproducible, stable and exhibited feasibility to determine Qn in tonic water with appreciable recovery percentage.
Dar et al. studied the electrochemical behavior of Qn in presence of different surfactants viz., Tween-20, sodium dodecylbenzenesulfonate (SDBS) and cetyltrimethylammonium bromide (CTAB) using CV and SWV.59 As compared to Tween-20 and SDBS, addition of CTAB to the solution containing Qn exhibited appreciable enhancement in reduction peak current, a greater shift in cathodic peak potential and a lower detection limit. The electrochemical process was diffusion-controlled and pseudo reversible at hanging mercury drop electrode (HMDE). The method displayed excellent accuracy, precision and reproducibility. No interferences from excipients such as gelatin, lactose, starch and magnesium stearate were observed. Further, the method was validated for the quantitative analysis of Qn in bark of Cinchona officinalis, commercial soft drinks (bitter lemon) and pharmaceutical formulations containing Qn. However, use of HMDE limits the use of the method. The electrode is bulky and involves potential risks of toxicity, contamination, poisoning and disposal associated with the use of metallic mercury. A bismuth-coated screen-printed carbon electrode (Bi-SPCE) was fabricated for voltammetric determination of Qn in tonic water.60 The main drawback of the method was that a fresh Bi-SPCE was required for every measurement. Later, a nanocomposite based on calf thymus dsDNA, methylene blue (MB) and MWCNTs was coated onto the GCE surface to fabricate dsDNA–MB–MWCNTs/GCE for enantioselective recognition of Qn and quinidine.61 The electrode was simple to prepare, stable as well as reproducible. However, the effect of interferents was not studied. Also, the method was not employed for quantitative analysis of the drug in real samples. Orata et al. reported the use of polyaniline and bentonite modified carbon electrode in 1 M H2SO4 to study the redox profiles of Qn in cinchona bark and malbet (a herbal medicine) using cyclic voltammetry.62 Later, the same group explored the electrochemical behaviour of Qn at polyaniline, bentonite and Qn modified carbon graphite electrode investigating the redox interaction of Qn with consumables (tea and milk), proteins/drugs (acetylsalicylic acid, tyrosine, cholesterol, leucine, paracetamol, iso-nicotinic acid, hydrocortisone and ferrous fumarate) and metal cations (Cu2+, Co2+, Zn2+ and Sn2+).63 However, in both the above-stated methods, quantitative analysis of Qn was not taken into consideration, with the work being primarily focussed on investigating the electrochemical behaviour of quinine using the modified electrodes.
The first potentiometric determination of Qn was reported by Kalman et al. using an ion-selective membrane electrode where the drug was quantified in pharmaceutical preparations.64 The proposed method provides an accuracy of 2.5%. Later, a picrate ion-selective electrode was fabricated for determination of micro-amounts of Qn along with other alkaloids in bulk as well as in pharmaceutical preparations.65 Anzai et al. investigated the performance characteristics of a Qn-sensitive membrane electrode based on the ion-association complex of Qn with tetraphenylborate (TPB) and tested its efficacy for determination of Qn in pharmaceutical preparations.66 The electrodes displayed good selectivity towards Qn in presence of various potentially interfering inorganic and organic ions. However, alkaloids such as papaverine, quinidine and yohimbine showed serious interference. Another potentiometric technique using a silver sulphide ion-selective electrode was proposed for the determination of Qn and some other drugs/physiologically active substances.67 Saad et al. used wall-jet flow-through potentiometric flow injection analysis (FIA) detectors to test the efficiency of the developed membranes for analysis of the drug.68,69 Qn selective sensors were fabricated based on a lipophilic ion-exchanger potassium tetrakis[3,5-bis(trifluoromethylphenyl)]borate (PTFB) in PVC membranes using dioctyl phthalate (DOP), 2-nitrophenyl phenyl ether (NPPE), and bis(2-ethylhexyl)adipate (BEHA) as plasticizers. The best performance was exhibited by the sensor containing BEHA. It was sensitive, fast-responding and exhibited near Nernstian response under batch injection analysis (BIA). Negligible interference by foreign species such as alkali and alkaline earth metal ions, sodium benzoate and sugars was noted. Analytical applicability of the method was evaluated by determining Qn in spiked mineral water samples as well as other drinks (carbonated and bitter lemon drinks) yielding satisfactory results.
A TPB in PVC matrix based sensor was developed using different borate derivatives, KTCPB, potassium tetrakis[3,5-bis(trifluoromethyl)phenyl] borate (KTFPB) and NaTPB as the ion-exchangers and 2-nitrophenyl octyl ether (NPOE), dioctyl sebacate (DOS) and didodecyl phthalate (DDP) as plasticizers.70 Selectivity measurements revealed no interference from metal cations (NH4+, K+, Na+ and Ca2+), ephedrine, glycine and glutamic acid. The membrane fabrication in the proposed method did not require separate preparation of the Qn-borate ion-pair, which was an improvement over other earlier reported potentiometric methods. This was attributed to the fact that the QuH+ cation, being more lipophilic than Na+ or K+ ions, could easily displace them from the sensing membrane. The KTCPB/DDP/PVC electrode gave the best response in terms of sensitivity and selectivity, and was successfully employed to determine Qn in tonic water as well as in orange, apple and white grape juices. The results obtained using standard addition technique showed high recovery and a low standard deviation suggesting the applicability of the method to determine Qn in coloured and benzoate containing substances. Molecular imprinted polymer technology was employed by Kamel et al. to develop miniaturized planar PVC based polymeric membrane sensors containing Qn-MAA and/or acrylic acid (AA)–ethylene glycol methacrylate (EGMA) followed by application of the method for routine determination of Qn in soft drinks.71 PVC membrane based silver (Ag) and copper (Cu) coated wire electrodes (CWEs) were constructed to determine quininium cation (Qn+).72 Ion-pairs and ion associates of Qn+ with reineckate (Rn−), phosphotungstate (PT3−) and phosphomolybdate (PM3−) were used as the ion-exchangers. Ag-Qn3PM CWE displayed best performance exhibiting appreciable selectivity, a Nernstian response along with good accuracy and precision. The method was then validated for micro determination of Qn2SO4 in pharmaceutical compounds containing Qn. The method does not give any information regarding detection limit. Also, gradual leaching of the electroactive ion-exchanger from the electrode membrane surface was observed leaving it soaked for a day. Solid state CWEs possess the advantage of being more easily prepared as compared to the conventional electrodes. Also, it does not require an internal reference electrode.
A novel potentiometric technique, differential dynamic potentiometry (DiDP), was proposed to study the effect of β-cyclodextrin (β-CD) on the response of electrodes sensitive to Qn and other pharmaceutical drugs.73 DiDP method consists of recording the dynamic potential difference between two ISEs. The responses in serial calibration mode were found to be characteristic for each individual drug including Qn. The effect of membrane plasticizers, 2-fluoro-2′-nitrodiphenyl ether (FNDPE), DOS, NPOE and tricresyl phosphate (TCP), on the electrode performance was monitored, with the TCP containing membrane showing the best response. The method was further used for quantitative analysis of Qn upto 1.0 mM. However, the selectivity and usability of the method in real samples was not investigated.
The electro-reduction behavior and quantification of MQ was studied in BR buffer at a HMDE using CV, DPV and SWV techniques.76 The analytical applicability of the method was verified in pharmaceutical dosage forms and biological samples. The method was fully validated and there was no possible interference from the excipients and endogenous substances found in tablets and biological fluids. A MQ ion-selective electrode was developed to determine the drug in blood plasma samples.77 High MQ protein binding resulted in reduced drug sensitivity and hence, poor potentiometric response was noted. Also, the method involved sample extraction prior to analysis of the drug in blood sample.
The cyclic voltammetric behaviour of AQ was investigated at a bare and DNA modified CPE in BR buffer (pH 4.0) studying the dynamics of DNA mediation in the transfer of electrons.80 Well-defined anodic and cathodic peaks were obtained at both the electrodes. Though there was not much variation in peak potential, a remarkable improvement in peak current was observed at DNA modified CPE. Presence of guanine interfered with the voltammetric response of AQ. Analytical applicability of the method in real samples was not validated. Also, the detection limit, stability, accuracy and reproducibility of the electrode were not reported. Later, Valente et al. developed a hemin-based electrode for voltammetric determination of the drug.81 The electrooxidation process was irreversible and diffusion-controlled. The method was then validated for analysis of AQ in breast milk without any prior sample treatment.
Eight PVC membrane ion-sensitive electrodes were fabricated and investigated for potentiometric determination of AQ hydrochloride.82 The sensing membrane was based on water-insoluble ion-pair complex formed between the cationic drug and NaTPB or KTCPB as the ion-exchanger. Various plasticizers namely, DOP, NPOE, bis(2-ethylhexyl)adipate (EHA) and dioctyl phenylphosphonate (DOPP) were investigated for fabricating the sensor and their performances were compared. A stable, fast and near-Nernstian response over a wide drug concentration range was exhibited by all the sensors. The overall best performance was exhibited when KTCPB and DOP was used as the ion-exchanger and plasticizer, respectively. Upon evaluating the selectivity of the sensor, CQ, Qn, imipramine, promethazine and alkaloids were found to show serious interference on the potentiometric response of AQ. The drug was successfully determined in tablets and in the reconstituted powder without any sample treatment.
Arguelho et al. investigated the electrochemical behavior of PQ in B–R buffer at a glassy carbon electrode (GCE) using linear, differential-pulse and square-wave voltammetry.84 The electron transfer process was suggested to involve dimerization of the product of charge transfer. It was proposed that the electrochemical oxidation of PQ in aqueous solution involved the quinolinic ring. The method was then successfully validated for determination of the drug in pharmaceutical samples without any extraction steps. The observed detection limit was high (in millimolar range) and the method did not test the applicability of the electrode in human body fluids. Later, a differential pulse voltammetric method was presented for quantification of PQ at Cu-NW/CPE in tablets exhibiting a good recovery percentage.47 The electrode showed good reproducibility and excellent stability. No interference from sorbitol, lactose, Na+, and K+ was observed towards the current response of the drug. The feasibility of the method for analysis of Qn in pharmaceutical preparations was successfully validated.
Macrocyclic crown ethers based ion-selective electrodes were developed as potentiometric sensors by Saad et al. for the quantification of PQ.85 Three macrocyclic crown ethers, namely dibenzo (18-crown-6), dibenzo (24-crown-8) (DB24C8) and dibenzo (30-crown-10) (DB30C10) were considered for electrode fabrication. Near-Nernstian responses were observed at DB24C8- and DB30C10-based membranes indicating that crowns with large cavity sizes are able to effectively encapsulate the bulky cation of PQ. The developed electrode showed good usability for about 30 days. However, after this period the membrane components of the electrode start leaching that lead to a sluggish and noisier response. Slight interference on the electrode response was observed in presence of CQ, sulfadimide and sulfacetamide. Also, the feasibility of the method for determination of the drug in real samples was not evaluated.
3.2 Artemisinin and its derivatives
Artemisinin and its derivatives (Fig. 2) are considered as the most important antimalarial group of drugs owing to their rapid action, high efficacy, tolerability and limited resistance in malarial parasites. The derivatives include artesunate, artemether and dihydroartemisinin. They show a broad spectrum of activity and are effective against Qn resistant malaria parasites. At present, artemisinin based combination therapy (ACT) is recommended as the first-line of treatment for uncomplicated P. falciparum malaria, where one of the artemisinin derivatives is combined with a drug from a different class. These have the potential to delay antimalarial drug resistance when administered in combination with long-acting antimalarial drugs such as pyrimethamine or AQ. Available in oral, parenteral and suppository formulations, artemisinin and its derivatives exert their antimalarial effect on the ring-forming younger parasites reducing parasite numbers by a factor of approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per asexual cycle.89 All artemisinins possess an endoperoxide moiety. Their antimalarial activity is believed to be due to activation of the drugs within the food vacuole of the intra-erythrocytic stage of the parasite, where reductive cleavage of the peroxide bond by heme liberated during digestion of haemoglobin generates reactive oxygen radicals. This induces oxidative stress and alkylates heme and vital parasite proteins causing damage to the parasite.90 A recent study suggested that the artemisinin derivatives display antimalarial activity by inhibiting a P. falciparum-encoded sarcoplasmic-endoplasmic reticulum calcium ATPase.91
000 per asexual cycle.89 All artemisinins possess an endoperoxide moiety. Their antimalarial activity is believed to be due to activation of the drugs within the food vacuole of the intra-erythrocytic stage of the parasite, where reductive cleavage of the peroxide bond by heme liberated during digestion of haemoglobin generates reactive oxygen radicals. This induces oxidative stress and alkylates heme and vital parasite proteins causing damage to the parasite.90 A recent study suggested that the artemisinin derivatives display antimalarial activity by inhibiting a P. falciparum-encoded sarcoplasmic-endoplasmic reticulum calcium ATPase.91
The interaction of ARN and haemoglobin was studied at layer-by-layer haemoglobin/poly(vinyl sulfonate) film modified glassy carbon and silver electrode.92 The catalytic activity of the electrodes was suggested to be due to the breaking of the peroxide bond in ARN by the prosthetic group heme in haemoglobin. Though the report presented a new understanding for the antimalarial mechanism, it did not explore quantitative analysis of ARN. Debnath et al. reported a differential pulse polarographic (DPP) method for the determination of ARN. The method was simple, sensitive, precise and was successfully used to quantify ARN in a traditional Chinese herbal drug Artemisia annua L.93 However, the method used DME which has several limitations such as variation in the surface area of mercury (Hg) drop, higher charging current and high consumption of Hg. Also, Hg is poisonous and hence, it needs to be carefully handled. Later, the same group developed a DPV method to determine the drug in Artemisia annua extracts using a cobalt phthalocyanine modified CPE and a hemin modified electrode with Adeps neutralis (solid fat) as binder.94,95 A DPP method was further proposed to determine the amount of ARN in herbal tea preparation of Artemisia annua.96 The tea prepared without cooking but shaking for 15 minutes gave high concentration of ARN. No interference was observed in the tea extract to estimate the drug. The method was simple and did not require any special separation technique or derivatization of ARN. Gong et al. synthesized various glycosylated porphyrin metal complexes and employed as active material for developing amperometric sensor for ARN detection.97 The working electrodes were prepared by incorporating different glycosylated porphyrin metal complexes including [ZnT(o-glu)PPCl], [FeT(o-glu)PPCl], [MnT(o-glu)PPCl] and T(o-glu)PPH2 in gold nanoparticles/chitosan matrix film coated onto the GCE surface and their characteristic performance was investigated using amperometry. The electrode having [FeT(o-glu)PPCl] as the active material exhibited the best response in terms of selectivity and sensitivity. Among EDTA-Fe3+, Fe3+, CN−, H2O2 and artemisinic acid, only CN− was found to interfere in the amperometric response of ARN. The method was stable as well as reproducible, and was successfully assessed for direct determination of ARN in plant extract samples.
The electrochemical behaviour of ARN was investigated at MWCNTs and dihexadecyl hydrogen phosphate modified glassy carbon electrode (MWCNTs–DHP/GCE) using CV and linear sweep voltammetry (LSV).98 No interference from other species (metal ions, ascorbic acid, vitamin E, vitamin B6, vitamin A, uric acid, lysine, tryptophan, cysteine and indole-3-acetic acid) was found towards the determination of ARN. The method was successfully applied to determine the content of ARN in Artemisia annua L. An amperometric sensor based on hemin immobilized on titanium oxide modified silica (STH) was developed and applied for determination of ARN in the crude extracts of A. vulgaris L.99 The selectivity of the electrode for ARN was evaluated in presence of interferents such as EDTA-Fe3+, Fe3+, H2O2 and CN−. None of the mentioned interferents affected the amperometric response of the drug. The modified electrode showed good stability and reproducibility. Phukon et al. fabricated a sensitive and selective biosensor based on HRP immobilized natural polymer polyhydroxyalkanoate with gold nanoparticle composite on indium-tin oxide (PHA/AuNPs/HRP/ITO) for the determination of ARN in bulk and spiked human serum.100 Recently, a molecularly imprinted membrane based sensor was fabricated for analysis of ARN.101 The sensor was constructed by first modifying the GCE with graphene (G) and then assembled with ARN imprinted membrane (ART-MIM) using in situ polymerization technique using ethylene glycol dimethacrylate (EGDMA) as cross-linking agent and acrylamide (AM) as the functional monomer and. The ART-MIM/G/GCE sensor displayed higher selectivity towards ART as compared to its derivatives, dihydroartemisinin, artemether and artesunate. Also, a lower detection limit and a wider dynamic range were observed as compared to the earlier reported methods. The analytical applicability of the sensor was successfully validated for the determination of ART in A. annua L.101
Jain et al. investigated the electrochemical behaviour of ARTS at glassy carbon electrode in phosphate, BR and acetate buffer systems.104 The best response was observed in phosphate buffer system containing 1.0% CTAB. No interference was observed from commonly encountered pharmaceutical excipients and endogenous substances usually present in biological samples. The method was successfully applied for determination of ARTS drug in tablets and spiked human serum, urine and plasma samples. Later, the same group fabricated MWCNTs modified electrode (MWCNTs/GCE) for quantitative analysis of ARTS in bulk and commercial formulations.105 The method led to improved voltammetric parameters including the detection limit. Recently, a horseradish peroxidase immobilized graphene oxide–polyaniline film based sensor was developed for analysis of ARTS in pharmaceutical dosage forms and biological fluids without any potential pharmaceutical excipients as well as the body fluid matrix interferences.106
The electrochemical behaviour of ARM was first studied at a GCE in presence of hemin. Hemin was found to catalyze the reduction process of ARM. However, the quantitative analysis of the drug was not performed.108 Later, Debnath et al. investigated the polarographic behaviour of ARM at mercury electrode using DiPP.109 None of the excipients commonly present in tablets/capsules was found to influence the peak current response of the drug, suggesting good specificity of the method. The analytical applicability of the method was successfully evaluated for analysis of the drug in tablets and capsules. Although the method was simple, accurate and precise, it has the disadvantages associated with the use of a mercury electrode. Orata et al. chemically modified the carbon graphite working electrode surface with LF/ARM (coartem) for electrochemical study of the drugs.88 The results suggested that ARM possesses redox active moieties. The drug also interacted electrochemically with amino acids. Bentonite was also used as the host matrix in the modified electrode to analyse ARM. However, the bentonite matrix was found to completely inhibit the redox activity of the drug. The study did not attempt to evaluate the analytical applicability of the modified electrode for quantification of ARM.
3.3 Antifolates
Since their discovery in the 1940s, antimalarial antifolates remain an important class of drugs for prophylaxis and treatment of malaria, both as single agents as well as in combinations. These drugs function by interfering with folate metabolism thus disrupting the pathway critical for the survival of the malaria parasite.111 Though effective, antifolates may cause hematologic effects if administered for a prolonged period of time.112 Malaria haemolysis may lead to folic acid deficiency and megaloblastic anemia.113 The chemical structures of antimalarial antifolates are shown in Fig. 3.The electrochemical behaviour of PG was first studied using d. c. polarography in BR buffered media.115 A reduction wave was observed which was attributed to the reduction of the two azomethine centers on the monoprotonated biguanide group. The detection limit was found to be 0.05–0.1 μg mL−1. Gelatin, Triton X-100 and other substances having a half-wave potential within 100 mV of that of PG were found to interfere with the electrochemical response of the drug. Later, Smarzewska et al. developed a renewable silver amalgam film electrode for determination of PG in tablets and spiked human urine.116 Though it was observed that presence of atovaquone did not affect PG determination, the effect of other interferents (excipients commonly found in pharmaceutical preparations and endogenous substances present in biological fluid matrix) was not investigated.
A PYM modified carbon graphite electrode surface was fabricated to investigate the electrochemical response of the drug in absence and presence of bentonite as the host matrix.88 PYM was observed to undergo electro-oxidation in a 1e−/1H+ pathway. The presence of bentonite as the host matrix did not significantly affect the redox potential of the drugs. This was attributed to the size of drug molecules which hindered isomorphous exchange or substitution within the tetrahedral and octahedral sites in the clay montmorillonite. The electrochemical interaction of the drug with amino acids (methionine, arginine, tyrosine and leucine) was also investigated. However, the work did not involve quantitative analysis of the drug. The electrochemical oxidation mechanism of PYM was examined at a screen printed carbon electrode (SPCE) in different aqueous supporting electrolytes.118 Further, a dsDNA modified SPCE (dsDNA/SPCE) was fabricated and subsequently tested to determine the drug in 0.02 M phosphate buffer system. No interference towards the electrochemical response of PYM was observed from potential interfering substances present in biological fluids such as D-glucose, ascorbic acid, L-cysteine, uric acid and cysteine. The analytical applicability of the dsDNA/SPCE sensor was validated for the analysis of the drug in spiked human blood serum samples. SPCEs offer the advantage of being simple, economical, disposable, portable, versatile, and have mass production capability. Being disposable the problems associated with adsorption and fouling of the electrode surface by products of redox processes are also evaded. However, presence of organic solvents limits the use of SPCEs since these solvents may dissolve the insulate inks leading to decline in sensitivity and detection limit. Another drawback of the method employing SPCE is that the electrode had to be replaced after every measurement.119–121
3.4 Antimicrobials and others
Some well-known antimicrobials and a hydroxynaphthaquinone drug were also found to exhibit activity on malaria parasites in their blood or mosquito stages, or both. The chemical structures of these drugs are displayed in Fig. 4.A fast fourier adsorptive stripping voltammetric method was developed using a gold ultramicroelectrode for the determination of CM in flow injection systems.126 The method was accurate, precise, sensitive and successfully employed to analyse CM in capsules. Molecular imprinting is a process that creates selective binding sites in synthetic polymers. These molecular imprinted polymers are particularly beneficial for sensors since they selectively bind the analyte. The technique offers advantages such as low cost, ease of preparation, high chemical and mechanical stability. Zhang et al. fabricated a molecularly imprinted electrochemical sensor by stepwise electrodeposition of a MWCNTs layer and a thin molecularly imprinted sol–gel film onto the surface of a gold electrode (MIP/sol–gel/MWCNTs/Au electrode) for detection of CM.127 On comparing the response currents towards CM hydrochloride, erythromycin, oxacillin sodium monohydrate, benzylpenicillin potassium and streptomycin sulphate, the developed sensor displayed high selectivity towards CM demonstrating the highest response current. However, the effect of potential interferents (substances commonly present in biological matrix) was not assessed. The usability of the imprinted sensor for determination of the drug in human urine samples was successfully evaluated. The voltammetric behavior of CM hydrochloride and its phosphate salt was examined at a CPE using AdSV.128 The method was accurate, precise and successfully applied for determining the drug in pharmaceutical preparations (capsules and lotion) and in human urine samples. However, the effect of potential interferents on the voltammetric response of the drug was not reported.
A sensitive adsorptive stripping method was developed for trace analysis of DC at HMDE exhibiting a detection limit of 6 × 10−10 M.132 The method involves potential risks of contamination, poisoning and disposal associated with the use of metallic mercury. A nickel-modified glassy carbon electrode (Ni/GCE) was fabricated for amperometric detection of DC in a flow injection system.133 The electrode offered good reproducibility, stability, accuracy and was validated for analysis of DC in commercial pharmaceutical formulations (capsules). However, the effect of biologically common interferents was not investigated. The general electrocatalytic behaviour of Ni/GCE towards various compounds (amino acids, carbohydrates and hydroxylated compounds) was anticipated to interfere with detection of DC, thus limiting the selectivity of the modified electrode. It is implied that due to the same reason, Ni/GCE was not employed to quantify DC in biological fluid samples. More flow injection methods with amperometric detection were developed at anodized boron-doped diamond (BDD) thin film electrode and a gold rotating disk electrode (Au RDE) for the determination of DC in different pharmaceutical formulations.134,135 Shaidarova et al. fabricated a mixed-valence ruthenium oxide–ruthenium cyanide (RuO–RuCN) film modified GCE for determining DC under BIA and FIA conditions.136 The presence of adjuvants such as nystatin and hydrocortisone did not affect the determination of the drug. The analytical applicability of the method was successfully evaluated for analysis of DC in syrup. Though the modified electrode was stable for several months, it required acidic conditions and the film was destroyed if the pH was increased towards alkalinity. Conzuelo et al. developed the first disposable amperometric immunosensor for detection of DC in milk samples.137 The immunosensor was fabricated by immobilizing DC antibody (Ab) on the surface of protein G-functionalized magnetic beads (ProtG-MBs) and depositing the suspension on SPCE. A horseradish peroxidase (HRP)-labelled specific tracer was used for direct competitive immunoassay of the drug. The presence of Ca2+ ions in milk and the non-target antibiotics (enrofloxacin, sulfapyridine, cefapirin, ampicillin, amoxicillin and penicillin G) did not affect the performance of the magneto-immunosensor. Pencil graphite electrode (PGE) modified with Ppy and imprinted with DC was used to investigate the voltammetric behaviour of the antibiotic in BR buffer solutions.138 The MIP electrode was further employed to determine DC in pharmaceuticals. Presence of ciprofloxacin, a quinolone group antibiotic, did not affect the differential pulse voltammetric response of DC. The effect of additives commonly found in pharmaceutical formulations using MIP/PGE was not reported. Also, the observed detection limit was considerably high as compared to other reported voltammetric methods for DC quantification.
The first potentiometric study for determination of DC hydrochloride in pharmaceutical preparations was performed at a simple PVC membrane based ISE.139 The electrode showed a linear response with Nernstian slope over the range of 7.9 × 10−5 to 1.9 × 10−3 M and exhibited appreciable selectivity for the drug over a large number of organic substances and inorganic cations of biological importance. Pekli Novak et al. constructed DC-TPB ion pair containing PVC-based DC-sensitive electrodes for the analysis of the drug in pharmaceutical preparations (capsules).140,141 A conducting Ppy film based DC-selective sensor was prepared by immobilizing the polymer onto the surface of a DC ion-pair complex in PVC membrane coated GCE.142 Among the developed GCEs modified with different DC ion-pairs, viz. DC-TPB, DC-phosphotungstate and DC-silicotungstate, as the electroactive substance in PVC membrane having dibutylphthalate (DBP) as the plasticizer agent, DC-TPB incorporated electrode displayed the best response. The sensor was stable, reproducible, exhibited a near-Nernstian response and was successfully applied to determine the drug in pharmaceutical formulations. The selectivity of the sensor (GC/Ppy/PVC) towards different inorganic cations (Na+, K+, NH4+, Ca2+, Mg2+ and Al3+) and citric acid was also investigated. Kamel et al. developed a molecularly imprinted MAA based sensor for DC analysis in tablets and biological fluids.143 The MIP was constructed using DC as a template molecule, MAA and/or AM as a functional monomer and EGDMA as a cross-linking agent. The MIP was then incorporated in a PVC matrix to develop the sensor. The sensor showed high selectivity, sensitivity, accuracy, precision and exhibited a near-Nernstian response. Issa et al. demonstrated the potentiometric determination of DC in pharmaceutical preparations using DC-TPB ion-pair modified CPE with DBP as plasticizer.144 Both BIA and FIA modes were employed. The sensor exhibited a Nernstian response over a wide concentration range, a fast response time and high selectivity over a wide variety of inorganic cations, sugars and amino-acids. Different carbon paste compositions were evaluated. The best performance was obtained with carbon paste composition of 3% DC-TPB, 48.5% DBP and 48.5% graphite. The sensor was successfully applied for determination of DC in pharmaceutical formulations. However, the electrode displayed limited long term stability with a life span of only 5 days.
The important electrode characteristics and validation parameters associated with the significant electroanalytical methods (voltammetric and potentiometric) reported for the determination of various antimalarial drugs have been summarized in Tables 3 and 4. Comparing these parameters, it becomes easier to select the more sensitive and versatile method.
| Drug | Electrode | Method | LCR (M) | LOD (M) | LOQ (M) | Matrix | Ref. |
|---|---|---|---|---|---|---|---|
| Chloroquine | dsDNA/CPE | DPV | 1.0 × 10−7 to 1.0 × 10−5 | 3.00 × 10−8 | — | Spiked human serum | 46 |
| Cu-NW/CPE | DPV | 2.1 × 10−7 to 2.1 × 10−5 | 3.10 × 10−8 | — | Pharmaceutical formulations | 47 | |
| Quinine | MWCNTs–RTIL/GCE | SWV | 3.0 × 10−6 to 1.0 × 10−4 | 0.44 × 10−6 | — | Pharmaceutical formulations | 55 |
| Ppy-PCNFe/Pt | CV | 1.0 × 10−3 to 9.0 × 10−3 | 1.08 × 10−5 | — | — | 56 | |
| p-(AHNSA)/GCE | SWV | 0.1 × 10−6 to 1.0 × 10−5 | 1.42 × 10−8 | — | Spiked human urine; pharmaceutical formulations | 57 | |
| EGMRA-MIP/GCE | Amperometry | 8.0 × 10−7 to 2.6 × 10−4 | 2.00 × 10−8 | — | Tonic water | 58 | |
| HMDE in presence of 1% CTAB | DPV, SWV | 27.7 × 10−8 to 19.4 × 10−7, 9.2 × 10−8 to 64.7 × 10−8 | 7.34 × 10−10, 4.07 × 10−10 | 2.44 × 10−9, 1.36 × 10−9 | Spiked plant extract (bark of cinchona officinalis); spiked soft drink; pharmaceutical formulations | 59 | |
| Mefloquine | HMDE | SWV | 6.0 × 10−6 to 6.0 × 10−5 | — | 4.50 × 10−7 | Spiked human serum and urine; pharmaceutical formulations | 76 |
| Amodiaquine | Hemin/GCE | CV | — | 9.27 × 10−6 | 3.09 × 10−5 | Breast milk | 81 |
| Primaquine | GCE | LSV, DPV, SWV | 3.0 × 10−5 to 1.0 × 10−2, 3.0 × 10−5 to 1.0 × 10−4, 3.0 × 10−5 to 1.0 × 10−4 | 3.62 × 10−5, 1.62 × 10−5, 6.94 × 10−6 | — | Pharmaceutical formulations | 84 |
| Cu-NW/CPE | DPV | 22.4 × 10−7 to 22.7 × 10−6 | 9.64 × 10−7 | — | Pharmaceutical formulations | 47 | |
| Artemisinin | DME | DPP | 6.4 × 10−7 to 3.2 × 10−5 | 2.05 × 10−7 | — | Plant extracts (Artemisia annua L.) | 93 |
| Hemin modified carbon fat electrode | DPV | 4.8 × 10−6 to 7.8 × 10−5 | 1.40 × 10−6 | — | Plant extracts (Artemisia annua) | 94 | |
| Cobalt phthalocyanine modified CPE | DPV | 2.1 × 10−5 to 5.3 × 10−4 | 6.50 × 10−6 | — | Plant extracts (Artemisia annua) | 95 | |
| DME | DPP | 63.8 × 10−8 to 31.9 × 10−6 | — | — | Herbal tea preparation of Artemisia annua | 96 | |
| [FeT(o-glu)PPCl]/AuNPs/GCE | Amperometry | 1.8 × 10−7 to 1.7 × 10−9 | 1.70 × 10−9 | — | — | 97 | |
| MWCNTs/DHP/GCE | LSV | 14.2 × 10−7 to 14.2 × 10−5 | 3.54 × 10−7 | — | Plant extracts (Artemisia annua L.) | 98 | |
| CPE/STH | Amperometry | 5.0 × 10−8 to 1.0 × 10−6 | 1.50 × 10−8 | 5.20 × 10−8 | Plant extracts (Artemisia vulgaris L.) | 99 | |
| PHA/AuNPs/HRP/ITO bio-electrode | DPV | 35.4 × 10−9 to 28.3 × 10−8 | 1.20 × 10−8 | 3.90 × 10−8 | Spiked human serum | 100 | |
| ART-MIM/G/GCE | DPV | 1.0 × 10−8 to 4.0 × 10−5 | 2.00 × 10−9 | — | Plant extracts (Artemisia annua L.) | 101 | |
| Artesunate | GCE in presence of CTAB | DPV, SWV | 10.4 × 10−6 to 10.4 × 10−5, 10.4 × 10−6 to 10.4 × 10−5 | 1.28 × 10−6 to 1.03 × 10−5, 8.84 × 10−8 to 6.37 × 10−7 | — | Spiked human serum, plasma and urine; pharmaceutical formulations | 104 |
| MWCNTs/GCE | DPV, SWV | 5.2 × 10−6 to 5.2 × 10−5, 5.2 × 10−6 to 5.2 × 10−5 | 1.40 × 10−7, 6.48 × 10−9 | 4.73 × 10−7, 2.15 × 10−8 | Pharmaceutical formulations | 105 | |
| GrO/PANI/HRP/ITO | SWV | 1.3 × 10−10 to 10.4 × 10−10 | 3.12 × 10−11 to 8.11 × 10−11 | — | Spiked human urine, serum and plasma; pharmaceutical formulations | 106 | |
| Artemether | DME | DPP | 3.4 × 10−7 to 3.0 × 10−5 | 1.07 × 10−7 | — | Pharmaceutical formulations | 109 |
| Dihydroartemisinin | SWCNTs/GCE | LSV | 5.0 × 10−7 to 5.0 × 10−4 | 3.00 × 10−7 | — | — | 110 |
| Proguanil | Hg(Ag)FE | SWV | 1.0 × 10−7 to 6.0 × 10−6 | 2.90 × 10−8 | 9.7 × 10−8 | Spiked human urine; pharmaceutical formulations | 116 |
| Pyrimethamine | DNA/SPCE | DPV | 1.0 × 10−7 to 5.0 × 10−5 | 1.00 × 10−8 | — | Spiked human serum | 118 |
| Sulfadoxine | G/PANI/SPCE | Amperometry | 3.2 × 10−8 to 3.2 × 10−5 | 9.65 × 10−9 | 3.22 × 10−8 | Shrimp | 123 |
| Clindamycin | Au ultra-microelectrode | ASV | 9.4 × 10−12 to 1.0 × 10−4 | 3.06 × 10−12 | 9.4 × 10−12 | Pharmaceutical formulations | 126 |
| MIP/sol–gel/MWCNTs/Au | SWV | 5.0 × 10−7 to 8.0 × 10−5 | 2.44 × 10−8 | — | Spiked human urine | 127 | |
| CPE | DPAdSV | 2.02 × 10−7 to 10.1 × 10−7 | 7.67 × 10−8 | 2.55 × 10−7 | Spiked human urine; pharmaceutical formulations | 128 | |
| Doxycycline | Ni/GCE | FIA | 5.62 × 10−6 to 22.5 × 10−5 | 2.07 × 10−6 | — | — | 133 |
| Anodized BDD | FIA | 5.0 × 10−4 to 5.0 × 10−2 | 1.00 × 10−8 | — | Pharmaceutical formulations | 134 | |
| Au RDE | FIA | 1.0 × 10−6 to 1.0 × 10−4 | 1.00 × 10−6 | — | Pharmaceutical formulations | 135 | |
| RuO–RuCN/GCE | FIA | 5.0 × 10−3 to 1.0 × 10−6 | — | — | Pharmaceutical formulations | 136 | |
| DC-HRP on antiDC-ProtG-MBs/SPCE | Amperometry | 28.1 × 10−9 to 15.2 × 10−7 | 8.77 × 10−9 | — | Spiked undiluted milk | 137 | |
| MIPpy/PGE | DPV | 5.0 × 10−5 to 5.0 × 10−4 | 4.25 × 10−5 | 1.41 × 10−4 | Pharmaceutical formulations | 138 |
| Drug | Type of electrode/sensor | Electrode composition | LCR (M) | LOD (μM) | Slope (mV decade−1) | Working pH range | Response time (s) | Matrix | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a PVC membrane electrode: poly(vinyl chloride) membrane based ion-selective electrode.b MIP membrane modified GCE: quinine based molecularly imprinted polymer modified glassy carbon electrode.c Solid state electrodes (Ag-CWEs: silver coated wire electrodes; Cu-CWEs: copper coated wire electrodes).d Internal solid contact sensor (GC/Ppy/PVC): polypyrrole film immobilized glassy carbon electrode surface with a plasticized polyvinyl chloride membrane containing DC-TPB ion-pair.e DC-TPB ion pair modified CPE: doxycycline-tetraphenylborate ion-pair containing carbon paste electrode. | |||||||||
| Chloroquine | aPVC membrane electrode | BEHA, KTCPB, THF | 0.1 × 10−4 to 1.0 × 10−1 | 20.0 | 28.3 | — | — | Pharmaceutical formulations; synthetic mixtures (tablet, serum electrolyte and biological fluids) | 51 |
| Quinine | PVC membrane electrode | PVC, NaTPB, DBP, THF | 5.0 × 10−5 to 1.0 × 10−1 | — | 56.3 | 5.3–7.3 | — | — | 66 |
| PVC membrane electrode | PVC, KTCPB, DDP, THF | 0.1 × 10−4 to 1.0 × 10−2 | 6.3 | 58.9 ± 1.0 | 4.4–8.0 | 10 | Soft drinks | 70 | |
| bMIP membrane modified GCE | PVC, MAA, THF, PVC, AA–EGMA, THF | 4.0 × 10−6 to 1.0 × 10−2, 1.0 × 10−5 to 1.0 × 10−2 | 1.2, 8.2 | 61.3 to 55.7 | — | — | Soft drinks | 71 | |
| cSolid state electrodes (Ag-CWEs, Cu-CWEs) | QnRn, Qn3PT, Qn3PM, PVC, THF, DOP, Ag/Cu coated wire | 1.0 × 10−6 to 1.7 × 10−2 | — | 48.8 to 55.4 | 3.2–7.6 | 5 | Pharmaceutical formulations | 72 | |
| Amodiaquine | PVC membrane electrode | PVC, KTCPB, THF, DOP | 1.0 × 10−2 to 1.6 × 10−5 | 11.0 | 29.3 | 3.7–5.5 | <30 | Pharmaceutical formulations | 82 |
| Primaquine | PVC membrane electrode | PVC, KTCPB, DB24C8, DOPP | — | 1.0 | 34.1 | 3.5–10 | 30 | Pharmaceutical formulations | 85 |
| PVC, KTCPB, DB30C10, DOPP | 8.9 | 33.4 | 3.5–10 | 30 | |||||
| Doxycycline | dInternal solid contact sensor, (GC/Ppy/PVC) | PVC, NaTPB, DBP, THF, Ppy, GCE | 1.0 × 10−2 to 1.0 × 10−5 | 4.0 | 54.4 | 3.5–8.0 | <15 | Pharmaceutical formulations | 142 |
| eDC-TPB ion pair modified CPE | NaTPB, graphite, DBP, CPE | 19.9 × 10−6 to 31.9 × 10−6 | 13.3 | 62.30 | — | <8–10 | Pharmaceutical formulations | 144 | |
4. Recognition receptors based electrochemical analysis of antimalarial drugs
An electrochemical sensor is an analytical device that comprise of a chemical (molecular) recognition system (receptor) immobilized onto the potentiometric or voltammetric transducer. The analyte interacts selectively with the receptor molecules resulting in a change in its physical properties in such a way that the appending transducer generates an analytically useful signal. When the recognition receptor is of biological origin, the chemical sensor is referred to as a biosensor. Recognition receptors play a vital role in the development of sensors. The recognition process is governed primarily by the shape and size of the receptor pocket and the analyte of interest. The function of the receptor is usually achieved by fabricating a thin layer that responds to particular species or a group of substances.In a biosensor, biological recognition elements such as antibodies, enzymes, DNA and aptamers have been used as specific receptors to a target molecule. Among these, enzymes are the most common recognition receptors. Enzyme based electrochemical sensors are characterized by the specific binding capabilities and catalytic activity of enzymes. The analyte is recognised via enzyme inhibition or conversion of the analyte into a product. Enzyme products or by-products may be electroactive, and thus the changes in their activity may be monitored using amperometry. Other enzymes may produce or consume protons, and their activity can be studied through pH changes. Peroxidase is one of the most common enzyme labels for electrochemical biosensors. Horse radish peroxidase (HRP) was used to catalyze the oxidation of antimalarial drugs, ARTS and ARN, in presence of hydrogen peroxide.100,106 Enzymes are also used as labels attached to antigens, antibodies and oligonucleotides with a specific sequence providing affinity-based sensors. A direct competitive immunoassay using a tracer with HRP for the enzymatic labeling was performed for amperometric detection of DC.134 The drug was quantified by competitive binding between DC and a HRP-labeled specific tracer for binding sites of the captured antibodies immobilized on the surface of protein-functionalized magnetic beads. Aptamers are specific oligonucleic acid sequences (DNA or RNA) or peptide molecules that recognize specific ligands and bind to various target molecules ranging from small metal ions and drugs to large proteins with high affinity and specificity. DNA strands have been used as receptors for antimalarial drug detection since they possess rich functional active groups such as amino groups and carbonyl groups, and could bind with drug molecules through electrostatic interactions, hydrogen bond and hydrophobic interactions.46,61,80,118 Peptides are amino-acids sequences which hold possibility of being used as a receptor for antimalarial drug detection since it may, like DNA, form tertiary structures where the drug cations can bind. Similar to DNA, peptides can be synthesized and selected by automated routes in a combinatorial way, and hence, hold promise to function as effective recognition elements in electrochemical sensors for analysis of antimalarial drugs. Very few biological components have been employed as specific receptors to antimalarial drugs in electrochemical sensors. Lack of stability and reusability are the main drawbacks associated with their use. Also, biological receptors are labile, expensive and it is difficult to obtain and prepare them. Hence, there have been attempts to synthesize specific recognition elements as an alternative to bio-receptors.
Synthetic recognition layers are primarily developed using molecular imprinting technique, where specific recognition sites for a target molecule are easily molded in synthetic polymer networks. The technique is based on the formation of a complex between the template (analyte) and a functional monomer in presence of a cross-linker. After polymerization, when the template is removed it leaves behind specific recognition sites in the polymer matrix that are complementary in shape, size and chemical functionality to the template molecule. Thus, the molecularly imprinted polymers (MIPs) display high affinity to the template molecules and bind them selectively. MIPs have gained wide acceptance as new molecular recognition materials since they are less expensive, more stable, robust, temperature and pressure resistant, and can be easily tailored as compared to the biological receptors. Integration of MIP-based recognition elements with sensors is usually achieved by either in situ polymerization on the surface of transduction platform or by deposition onto the transducer surface as thin films. A number of MIPs have been prepared to suit a variety of templates and used in immunoassays, chemical sensors and biosensors. The technology has been implemented to electrochemically detect and quantify few antimalarial drugs, as discussed above. A MIP film was created on GCE surface using free radical polymerization method for determination of Qn.58 Kamel et al. built MIPs based potentiometric sensors for flow injection analysis of Qn and DC.71,140 A thin sol–gel film of MIPs was electrodeposited onto the surface of MWNT decorated gold electrode to fabricate an electrochemical sensor for selective determination of clindamycin.124 DC imprinted polypyrrole modified electrodes were formed on PGE and successfully used to determine the drug.135 ARN-imprinted membranes were polymerized on the surface of graphene modified GCE for sensitive analysis of ARN.140 Owing to their compatibility to highly complex matrices, MIPs have gained an increasing interest in the analysis of drugs and for biological estimations.
5. Sample preparation
The need for drug analysis in various biological fluids (serum, plasma and urine etc.) and pharmaceutical formulations is an important criteria for pharmacokinetic studies, investigation of therapeutic and toxic effects of the drug, and to determine the drug's physiological performance. Matrices of biological fluids and pharmaceutical products are complicated, containing a number of compounds such as proteins, acids, bases and organic compounds that may interfere in the analysis. Moreover, the analytes are usually present in low quantity in biological samples. Hence, sample preparation is of key importance in order to properly isolate the desired analyte from complex matrices since most of the analytical instruments cannot analyse the compound from the matrix with extreme specificity. The basic concept of sample-preparation methods is to convert a real matrix into a sample suitable for analysis. The procedure is expected to be fast, cheap, convenient, remove coexisting potential interfering components and able to recover a good amount of the analyte from the matrix with minimal sample loss. Various sample pre-treatments and extraction techniques have been employed for the determination of antimalarial dugs using different techniques. Human blood serum and plasma were mainly found to be extracted by solid phase extraction or liquid–liquid extraction, directly. Majority of the analytical techniques reported for determination of antimalarials involve chromatography method, where sample preparation is a prerequisite step for effective analysis of the drug in real samples. The present review focusses on electroanalytical methods for determination of antimalarial drugs. These methods are simple and do not involve lengthy, tedious and time-consuming sample preparation and extraction processes. Pharmaceutical formulation samples were directly analysed after dissolving in water or any other solvent, which was sometimes followed by filtration.47,55,57,59 Human serum and plasma samples were usually centrifuged to precipitate the proteins and then diluted with the buffer solution before carrying out the analysis.46,76,104,106 For urine, a simple dilution is applied as a pre-extraction step, which is often the only step performed.57,76,104 Rarely, centrifugation of urine was done as a sample pretreatment step.106 No pretreatment step was required for analysis of amodiaquine in breast milk using hemin based electrochemical sensor.816. Current challenges and future perspectives
Last two decades witnessed a sharp rise in electroanalytical methods for antimalarial drug analysis, particularly for determination of CQ, Qn, ARN, ARTS, CM and DC. However, there were limited publications with respect to electrochemical analysis of MQ, PQ, DHA, PG, SD and PYM. PRQ and ATQ still remain unexplored electroanalytically. Thus, there is a need for substantial electrochemical study and determination of these drugs for effective analysis in various real samples. Though significant progress was observed in voltammetric determination of different antimalarial drugs with excellent sensitivity and selectivity, the analytical applicability of the developed sensors mainly remained restricted to their use in pharmaceutical formulations. Very few voltammetric methods and only one potentiometric method were evaluated to check their feasibility for analysis of the target drug in biological fluids. Thus, there is still a dearth of efficacious electroanalytical techniques that determine antimalarial drugs in human body fluids, especially blood serum and plasma. Hence, the current challenge is to develop electrochemical sensors that determine the drugs in biological samples in order to enhance the utility of the technique for routine clinical analysis.Considering the constant need for portable instruments for screening in a cost-effective and clinically pertinent manner, the construction of miniaturized small sensors would further extend their applicability. Thus, the possibility of commercialization suggests a promising future for development of commercially-available electrochemical sensors for analysis of antimalarial drugs for field use.
7. Conclusion
The present review investigates the electrochemical techniques for determination of antimalarial drugs revealing the current status and trends in their development. The prominent voltammetric and potentiometric methods used to quantify the drugs in complex matrices, such as biological fluids, pharmaceutical formulations, plant extracts and different kinds of drinks including milk, are discussed. Voltammetric techniques were observed to be more predominantly used as compared to the potentiometric ones. Various electrode materials, especially nano-dimensional, were employed to modify the electrode surface and improve the selectivity and sensitivity of the electrochemical sensor. Use of conducting polymers, nanomaterials and presence of surfactants significantly amplified the electrochemical signal achieving a lower limit of detection (upto as low as picomolar range) in antimalarial drug assays. It is expected that the information provided in the review would aid in development of improved electrochemical sensors for sensitive and selective determination of antimalarial drugs, particularly in real samples.8. Abbreviations
| AA–EGMA | Acrylic acid–ethylene glycol methacrylate |
| Ag-CWEs | Silver coated wire electrodes |
| Anodized BDD | Anodized boron-doped diamond electrode |
| ART-MIM/G/GCE | Artemisinin-imprinted membranes on the surface of graphene modified glassy carbon electrode |
| ASV | Adsorptive stripping voltammetry |
| Au RDE | Gold rotating disk electrode |
| BEHA | Bis(2-ethylhexyl)adipate |
| Bi-SPCE | Bismuth film coated screen-printed carbon electrode |
| CPE/STH | Hemin immobilized on a titanium oxide modified silica based carbon paste electrode |
| CPE | Carbon paste electrode |
| CTAB | Cetyltrimethylammonium bromide |
| Cu-CWEs | Copper coated wire electrodes |
| Cu-NW/CPE | Cu(OH)2 nano-wire-modified carbon paste electrode |
| CV | Cyclic voltammetry |
| DB24C8 | Dibenzo(24-crown-8) |
| DB30C10 | Dibenzo(30-crown-10) |
| DBP | Dibutylphthalate |
| DC | Doxycycline |
| DC-HRP on antiDC-ProtG-MBs/SPCE | Horseradish peroxidase conjugated doxycycline on antibody immobilized on the surface of protein G functionalized magnetic beads based screen printed carbon electrode (immunosensor) |
| DDP | Didecyl phthalate |
| DHP | Dihexadecyl hydrogen phosphate |
| DME | Dropping mercury electrode |
| DNA/SPCE | DNA modified screen printed carbon electrode |
| dsDNA-MB-MWNTs/GCE | Calf thymus double stranded DNA, methylene blue and multiwall carbon nanotubes modified glassy carbon electrode |
| DOP | Dioctyl phthalate |
| DOPP | Dioctyl phenyl phosphonate |
| DPP | Differential pulse polarography |
| DPV | Differential pulse voltammetry |
| dsDNA/CPE | dsDNA-modified carbon paste electrode |
| EGMRA-MIP/GCE | Ethylene glycol maleic rosinate acrylate based molecularly imprinted polymer modified glassy carbon electrode |
| [FeT(o-glu)PPCl]/AuNPs/GCE | Fe(III) coordinating glycosylated porphyrin in gold nanoparticles-chitosan film modified glassy carbon electrode |
| FIA | Flow injection amperometry |
| G/PANI/SPCE | Graphene/polyaniline modified screen-printed carbon electrode |
| GC/Ppy/PVC | Polypyrrole film immobilized on a glassy carbon electrode in plasticized polyvinyl chloride membrane |
| GrO/PANI/HRP/ITO electrode | Graphene–polyaniline–horseradish peroxidase modified ITO electrode |
| Hg(Ag)FE | Silver amalgam film electrode |
| HMDE | Hanging mercury drop electrode |
| KTCPB | Potassium tetrakis(4-chlorophenyl) borate |
| LSV | Linear sweep voltammetry |
| MAA | Methacrylic acid |
| MIP/sol–gel/MWNT/Au | Multi-wall carbon nanotube layer with a thin molecularly imprinted sol–gel film modified gold electrode |
| MIPpy/PGE | Molecularly imprinted non-imprinted polypyrrole modified pencil graphite electrode |
| MWCNTs/GCE | Multi-walled carbon nanotubes modified glassy carbon electrode |
| MWCNTs–RTIL/GCE | Multiwall carbon nanotubes–room-temperature ionic liquid modified glassy carbon electrode |
| NaTPB | Sodium tetraphenylborate |
| Ni/GCE | Nickel-modified glassy carbon electrode |
| p-(AHNSA)/GCE | Poly(4-amino-3-hydroxynaphthalene sulfonic acid)-modified glassy carbon electrode |
| PHA/AuNPs/HRP/ITO | Horse-radish peroxidase immobilized polyhydroxyalkanoate–gold nanoparticle composite on indium-tin oxide electrode |
| Ppy-PCNFe/Pt | Polypyrrole-pentacyanoferrate modified platinum electrode |
| PVC | Polyvinyl chloride |
| Qn3PM | Ion pair of quininium and phosphomolybdate |
| Qn3PT | Ion pair of quininium and phosphotungstate |
| QnRn | Ion pair of quininium and reineckate |
| RuO–RuCN/GCE | Ruthenium oxide–ruthenium cyanide film on a glassy carbon electrode |
| SWCNTs/GCE | Single-walled carbon nanotubes modified glassy carbon electrode |
| SWV | Square wave voltammetry |
| TCP | Tricresyl phosphate |
| THF | Tetrahydrofuran |
Acknowledgements
The author (N. T.) is thankful to the Nanotechnology Platform, University of KwaZulu-Natal (UKZN) and College of Health Sciences, UKZN, Durban, South Africa for providing financial support.References
- World Health Organization, Malaria Fact sheet No. 94, 2015, http://www.who.int/mediacentre/factsheets/fs094/en/, accessed on 4 December, 2015.
- N. Tangpukdee, C. Duangdee, P. Wilairatana and S. Krudsood, Korean J. Parasitol., 2009, 47, 93–102 CrossRef PubMed.
- S. Yeung, W. Pongtavornpinyo, I. M. Hastings, A. J. Mills and N. J. White, Am. J. Trop. Med. Hyg., 2004, 71, 179–186 Search PubMed.
- O. N. Adedeji, O. O. Bolaji, C. O. Falade, O. A. Osonuga and O. G. Ademowo, Antimicrob. Agents Chemother., 2015, 59, 5114–5122 CrossRef CAS PubMed.
- V. F. Samanidou, E. N. Evaggelopoulou and I. N. Papadoyannis, J. Pharm. Biomed. Anal., 2005, 38, 21–28 CrossRef CAS PubMed.
- A. Lawal, R. A. Umar, M. G. Abubakar and U. Z. Faruk, Int. J. ChemTech Res., 2012, 4, 669–676 CAS.
- P. Singhal, A. Gaur, A. Gautam, B. Varshney, J. Paliwal and V. Batra, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 859, 24–29 CrossRef CAS PubMed.
- C. Z. Liu, H. Y. Zhou and Y. Zhao, Anal. Chim. Acta, 2007, 581, 298–302 CrossRef CAS PubMed.
- U. Hellgren, T. Villen and O. Ericsson, J. Chromatogr., 1990, 528, 221–227 CrossRef CAS PubMed.
- K. Govender, L. Gibhard, L. Du Plessis and L. Wiesner, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 2015, 985, 6–13 CrossRef CAS PubMed.
- M. T. Koesdjojo, Y. Wu, A. Boonloed, E. M. Dunfield and V. T. Remcho, Talanta, 2014, 130, 122–127 CrossRef CAS PubMed.
- B. N. Divya, N. Shetty and S. Samshuddin, J. Chem. Pharm. Res., 2012, 4, 1647–1653 Search PubMed.
- D. J. Bell, S. K. Nyirongo, M. E. Molyneux, P. A. Winstanley and S. A. Ward, J. Chromatogr. B Analyt. Technol. Biomed. Life Sci., 2007, 847, 231–236 CrossRef CAS PubMed.
- O. A. Farghaly, R. S. Abdel Hameed and A. A. H. Abu-Nawwas, Int. J. Electrochem. Sci., 2014, 9, 3287–3318 Search PubMed.
- M. M. Ghoneim, M. K. Abdel-Azzem, H. S. El-Desoky, A. M. Ghoneim and A. E. Khattab, J. Braz. Chem. Soc., 2014, 25, 1407–1418 CAS.
- N. Thapliyal, R. V. Karpoormath and R. N. Goyal, Anal. Chim. Acta, 2015, 853, 59–76 CrossRef CAS PubMed.
- Y. W. Shaojun Dong, Electroanalysis, 1989, 1, 99–106 CrossRef.
- L. Yang, L. Zou, G. Li and B. Ye, Talanta, 2016, 147, 634–640 CrossRef CAS PubMed.
- P. K. Brahman, N. Pandey, S. N. Topkaya and R. Singhai, Talanta, 2015, 134, 554–559 CrossRef CAS PubMed.
- R. R. Chillawar, K. K. Tadi and R. V. Motghare, J. Anal. Chem., 2015, 70, 399–418 CrossRef CAS.
- A. Lewenstam, Electroanalysis, 2014, 26, 1171–1181 CrossRef CAS.
- E. Bakker and E. Pretsch, Angew. Chem., 2007, 46, 5660–5668 CrossRef CAS PubMed.
- J. S. Rana, J. Jindal, V. Beniwal and V. Chhokar, J. Am. Sci., 2010, 6, 353–375 Search PubMed.
- O. Walker, A. H. Dawodu, A. A. Adeyokunnu, L. A. Salako and G. Alvan, Br. J. Clin. Pharmacol., 1983, 16, 701–705 CrossRef CAS PubMed.
- L. L. Gustafsson, B. Lindstrom, A. Grahnen and G. Alvan, Br. J. Clin. Pharmacol., 1987, 24, 221–224 CrossRef CAS PubMed.
- C. B. Braga, A. C. Martins, A. D. E. Cayotopa, W. W. Klein, A. R. Schlosser, A. F. da Silva, M. N. de Souza, B. W. B. Andrade, J. A. Filgueira-Junior, W. d. J. Pinto and M. da Silva-Nunes, Interdiscip. Perspect. Infect. Dis., 2015, 2015, 346853/1–346853/7 Search PubMed.
- J. Achan, A. O. Talisuna, A. Erhart, A. Yeka, J. K. Tibenderana, F. N. Baliraine, P. J. Rosenthal and U. D'Alessandro, Malar. J., 2011, 10, 144/1–144/12 CrossRef PubMed.
- World Health Organization, Guidelines for the Treatment of Malaria, April 2015, http://apps.who.int/iris/bitstream/10665/162441/1/9789241549127_eng.pdf?ua=1%<?pdb_no 26ua?><?pdb_no 26ua?>26ua<?pdb END?><?pdb<?db_id PDB?> END?>=1, accessed on 4 December, 2015.
- D. E. Schwartz, G. Eckert and J. M. Ekue, Chemotherapy, 1987, 33, 305–308 CrossRef CAS PubMed.
- H. Jewell, J. L. Maggs, A. C. Harrison, P. M. O'Neill, J. E. Ruscoe and B. K. Park, Xenobiotica, 1995, 25, 199–217 CrossRef CAS PubMed.
- G. W. Mihaly, S. A. Ward, G. Edwards, M. L. Orme and A. M. Breckenridge, Br. J. Clin. Pharmacol., 1984, 17, 441–446 CrossRef CAS PubMed.
- J. Tarning, T. Singtoroj, A. Annerberg, M. Ashton, Y. Bergqvist, N. J. White, N. P. Day and N. Lindegardh, J. Pharm. Biomed. Anal., 2006, 41, 213–218 CrossRef CAS PubMed.
- O. M. Minzi, I. A. Marealle, S. Shekalaghe, O. Juma, E. Ngaimisi, M. Chemba, M. Rutaihwa, S. Abdulla and P. Sasi, Malar. J., 2013, 12, 174/1–174/8 CrossRef PubMed.
- A. Djimde and G. Lefevre, Malar. J., 2009, 8, S4/1–S4/8 CrossRef PubMed.
- T. K. Dien, P. J. de Vries, N. X. Khanh, R. Koopmans, L. N. Binh, D. D. Duc, P. A. Kager and C. J. van Boxtel, Antimicrob. Agents Chemother., 1997, 41, 1069–1072 CAS.
- K. M. Matar, A. I. Awad and S. B. Elamin, Am. J. Trop. Med. Hyg., 2014, 90, 1087–1093 CrossRef CAS PubMed.
- K. Na Bangchang, J. Karbwang, C. G. Thomas, A. Thanavibul, K. Sukontason, S. A. Ward and G. Edwards, Br. J. Clin. Pharmacol., 1994, 37, 249–253 CrossRef CAS PubMed.
- P. Tan-ariya, K. Na-Bangchang, R. Ubalee, A. Thanavibul, P. Thipawangkosol and J. Karbwang, Southeast Asian J. Trop. Med. Public Health, 1998, 29, 18–23 CAS.
- A. A. Somogyi, H. A. Reinhard and F. Bochner, Br. J. Clin. Pharmacol., 1996, 41, 175–179 CrossRef CAS PubMed.
- A. Le Liboux, H. Duquesne, G. Montay, D. Chassard, E. Pichard, J. J. Thebault, A. Frydman and J. Gaillot, Eur. J. Drug Metab. Pharmacokinet., 1991, 3, 284–290 Search PubMed.
- H. Xie, H. Chen, Y. Hu, S. Xu, Q. He, J. Liu, W. Hu and Z. Liu, Am. J. Nephrol., 2013, 38, 179–183 CrossRef CAS PubMed.
- K. N. Agwuh and A. MacGowan, J. Antimicrob. Chemother., 2006, 58, 256–265 CrossRef CAS PubMed.
- G. L. Nixon, D. M. Moss, A. E. Shone, D. G. Lalloo, N. Fisher, P. M. O'Neill, S. A. Ward and G. A. Biagini, J. Antimicrob. Chemother., 2013, 68, 977–985 CrossRef CAS PubMed.
- L. W. Kitchen, D. W. Vaughn and D. R. Skillman, Clin. Infect. Dis., 2006, 43, 67–71 CrossRef CAS PubMed.
- V. R. Solomon and H. Lee, Eur. J. Pharmacol., 2009, 625, 220–233 CrossRef CAS PubMed.
- A. Radi, Talanta, 2005, 65, 271–275 CAS.
- M. H. Mashhadizadeh and M. Akbarian, Talanta, 2009, 78, 1440–1445 CrossRef CAS PubMed.
- V. V. Cosofret and R. P. Buck, Anal. Chim. Acta, 1985, 174, 299–303 CrossRef CAS.
- S. S. Hassan and M. A. Ahmed, J. - Assoc. Off. Anal. Chem., 1991, 74, 900–905 CAS.
- B. Saad, K. Kanapathy, M. N. Ahmad, A. H. Hussin and Z. Ismail, Talanta, 1991, 38, 1399–1402 CrossRef CAS PubMed.
- B. Saad, M. Z. Zin, M. S. Jab, I. A. Rahman, M. I. Saleh and S. Mahsufi, Anal. Sci., 2005, 21, 521–524 CrossRef CAS PubMed.
- P. Cettour-Rose, C. Bezençon, C. Darimont, J. le Coutre and S. Damak, BMC Physiol., 2013, 13, 1–11 CrossRef PubMed.
- J. Thomas and H. P. Pietsch, Lebensmittelindustrie, 1990, 37, 29–30 CAS.
- C. Apetrei, M. L. Rodríguez-Méndez, V. Parra, F. Gutierrez and J. A. de Saja, Sens. Actuators, B, 2004, 103, 145–152 CrossRef CAS.
- X. M. Zhan, L. H. Liu and Z. N. Gao, J. Solid State Electrochem., 2011, 15, 1185–1192 CrossRef CAS.
- S. Awasthi, A. Srivastava and M. L. Singla, Synth. Met., 2011, 161, 1707–1712 CrossRef CAS.
- A. Geto, M. Amare, M. Tessema and S. Admassie, Anal. Bioanal. Chem., 2012, 404, 525–530 CrossRef CAS PubMed.
- L. Liu, X. Tan, X. Fang, Y. Sun, F. Lei and Z. Huang, Electroanalysis, 2012, 24, 1647–1654 CrossRef CAS.
- R. A. Dar, P. K. Brahman, S. Tiwari and K. S. Pitre, Colloids Surf., B, 2012, 98, 72–79 CrossRef CAS PubMed.
- A. Alberich, N. Serrano, J. M. Díaz-Cruz, C. Ariño and M. Esteban, J. Chem. Educ., 2013, 90, 1681–1684 CrossRef CAS.
- Q. Zhang, Y. Huang, L. Guo, C. Chen, D. Guo, Y. Chen and Y. Fu, New J. Chem., 2014, 38, 4600–4606 RSC.
- D. Orata, Y. Amir, C. Nineza, D. Mbui and M. Mukabi, IOSR J. Appl. Chem., 2014, 7, 81–89 CrossRef CAS.
- D. Orata, Y. Amir and C. Nineza, IOSR J. Appl. Chem., 2014, 7, 56–72 CrossRef CAS.
- J. Kalman, K. Toth and D. Küttel, Acta Pharm. Hung., 1971, 41, 267–272 CAS.
- E. P. Diamandis and T. P. Hadjiioannou, Anal. Chim. Acta, 1981, 123, 341–345 CrossRef CAS.
- J. Anzai, C. Isomura and T. Osa, Chem. Pharm. Bull., 1985, 33, 236–241 CAS.
- S. I. Obtemperanskaya, M. M. Buzlanova, I. V. Karandki, R. Shakhid and A. N. Kashin, J. Anal. Chem., 1996, 51, 419–423 CAS.
- B. Saad, Y. Bee-Leng and M. I. Saleh, Lab. Rob. Autom., 2000, 12, 133–137 CrossRef CAS.
- B. Saad, Y. Bee-Leng, M. I. Saleh, I. A. Rahman and S. M. Mansor, J. AOAC Int., 2001, 84, 1151–1157 CAS.
- M. M. Zareh, E. Malinowska and K. Kasiura, Anal. Chim. Acta, 2001, 447, 55–61 CrossRef CAS.
- A. H. Kamel and H. E. Sayour, Electroanalysis, 2009, 21, 2701–2708 CrossRef CAS.
- L. A. Al Shatti, H. M. Marafie and A. F. Shoukry, Am. J. Appl. Sci., 2011, 8, 116–123 CrossRef CAS.
- M. Cuartero, J. A. Ortuño, M. S. García and F. Martínez-Ortiz, Electrochim. Acta, 2012, 69, 152–159 CrossRef CAS.
- R. L. Nevin, Int. J. Parasitol.: Drugs Drug Resist., 2014, 4, 118–125 CrossRef PubMed.
- P. Schlagenhauf, M. Adamcova, L. Regep, M. T. Schaerer and H. G. Rhein, Malar. J., 2010, 9, 357/1–357/15 CrossRef PubMed.
- B. Uslu, B. Dogan, A. Sibel, H. Y. Özkan and Y. Aboul-Enein, Electroanalysis, 2005, 17, 1563–1570 CrossRef CAS.
- D. W. Mendenhall, T. Higuchi and L. A. Sternson, J. Pharm. Sci., 1979, 68, 746–750 CrossRef CAS PubMed.
- C. C. Campbell, D. Payne, I. K. Schwartz and O. J. Khatib, Am. J. Trop. Med. Hyg., 1983, 32, 1216–1220 CAS.
- L. N. Markham, E. Giostra, A. Hadengue, M. Rossier, M. Rebsamen and J. Desmeules, Am. J. Trop. Med. Hyg., 2007, 77, 14–15 CAS.
- M. L. P. M. Arguelho, J. P. H. Alves, N. R. Stradiotto, V. Lacerda Jr, J. M. Pires and A. Beatriz, Quim. Nova, 2010, 33, 1291–1296 CrossRef CAS.
- C. O. Valente, C. A. B. Garcia, J. P. H. Alves, M. V. B. Zanoni, N. R. Stradiotto and M. L. P. Arguelho, ECS Trans., 2012, 43, 297–304 CAS.
- T. K. Malongo, B. Blankert, O. Kambu, K. Amighi, J. Nsangu and J. M. Kauffmann, J. Pharm. Biomed. Anal., 2006, 41, 70–76 CrossRef CAS PubMed.
- D. Fernando, C. Rodrigo and S. Rajapakse, Malar. J., 2011, 10, 351/1–351/12 CrossRef PubMed.
- M. L. P. M. Arguelho, M. V. B. Zanoni and N. R. Stradiotto, Anal. Lett., 2005, 38, 1415–1425 CrossRef CAS.
- B. B. Saad, Z. A. Zahid, S. A. Rahman, M. N. Ahmad and A. H. Husin, Analyst, 1992, 117, 1319–1321 RSC.
- N. Gargano, F. Cenci and Q. Bassat, Trop. Med. Int. Health, 2011, 16, 1466–1473 CrossRef CAS PubMed.
- Q. Bassat, PLoS Neglected Trop. Dis., 2011, 5, e1325/1321–e1325/1310 Search PubMed.
- D. Orata, Y. Amir and C. Nineza, J. Chem. Chem. Eng., 2014, 8, 215–225 CAS.
- F. Nosten and N. J. White, Am. J. Trop. Med. Hyg., 2007, 77, 181–192 CAS.
- S. Krishna, A. C. Uhlemann and R. K. Haynes, Drug Resist. Updates, 2004, 7, 233–244 CrossRef CAS PubMed.
- A. Fernandez-Martinez, P. Mula, P. Cravo, P. Charle, A. Amor, P. Ncogo, A. Benito and P. Berzosa, Am. J. Trop. Med. Hyg., 2013, 88, 43–47 CrossRef CAS PubMed.
- P. H. Yang, Z. J. Zhou and J. Y. Cai, Colloids Surf., A, 2005, 257–258, 467–472 CrossRef CAS.
- C. Debnath, E. Haslinger and A. Ortner, Nat. Prod. Commun., 2006, 1, 487–494 CAS.
- C. Debnath, P. Saha and A. Ortner, Electroanalysis, 2008, 20, 1549–1555 CrossRef CAS.
- C. Debnath, P. Saha and A. Ortner, Electroanalysis, 2009, 21, 657–661 CrossRef CAS.
- C. Debnath, A. Dobernig, P. Saha and A. Ortner, J. Korean Chem. Soc., 2011, 55, 57–62 CrossRef CAS.
- F. C. Gong, Z. D. Xiao, Z. Cao and D. X. Wu, Talanta, 2007, 72, 1453–1457 CrossRef CAS PubMed.
- X. Yang, T. Gan, X. Zheng, D. Zhu and K. Wu, Bull. Korean Chem. Soc., 2008, 29, 1386–1390 CrossRef CAS.
- J. R. M. Reys, P. R. Lima, A. G. Cioletti, A. S. Ribeiro, F. Caxico de Abreu, M. O. F. Goulart and L. T. Kubota, Talanta, 2008, 77, 909–914 CrossRef CAS.
- P. Phukon, K. Radhapyari, B. K. Konwar and R. Khan, Mater. Sci. Eng., C, 2014, 37, 314–320 CrossRef CAS PubMed.
- H. Bai, C. Wang, J. Chen, J. Peng and Q. Cao, Biosens. Bioelectron., 2015, 64, 352–358 CrossRef CAS PubMed.
- Q. Li and P. Weina, Pharmaceuticals, 2010, 3, 2322–2332 CrossRef CAS.
- A. K. Das, Ann. Med. Health Sci. Res., 2015, 5, 93–102 CrossRef CAS PubMed.
- R. Jain and Vikas, Colloids Surf., B, 2011, 88, 729–733 CrossRef CAS PubMed.
- R. Jain and Vikas, Chem. Senses, 2011, 1, 15/1–15/8 CAS.
- K. Radhapyari, P. Kotoky, M. R. Das and R. Khan, Talanta, 2013, 111, 47–53 CrossRef CAS PubMed.
- L. Cui and X. Z. Su, Expert Rev. Anti-Infect. Ther., 2009, 7, 999–1013 CrossRef CAS PubMed.
- Y. Chen, J. Electrochem. Soc., 1997, 144, 1891–1894 CrossRef CAS.
- C. Debnath, E. Haslinger, W. Likussar and A. Michelitsch, J. Pharm. Biomed. Anal., 2006, 41, 638–643 CrossRef CAS PubMed.
- H. Wang, P. Yang, S. Liang and J. Cai, Yaowu Fenxi Zazhi, 2010, 30, 431–434 CAS.
- A. Saifi, Afr. J. Pharm. Pharmacol., 2013, 7, 148–156 CrossRef.
- S. Waxman and V. Herbert, N. Engl. J. Med., 1969, 280, 1316–1319 CrossRef CAS PubMed.
- G. T. Strickland and J. E. Kostinas, Am. J. Trop. Med. Hyg., 1970, 19, 910–915 CAS.
- J. E. Hyde, Acta Trop., 2005, 94, 191–206 CrossRef CAS PubMed.
- F. Vicente-Pedros, F. Tomas-Vert and J. Vera-Sanchez, J. Pharm. Sci., 1984, 73, 1804–1806 CrossRef CAS PubMed.
- S. Smarzewska, S. Skrzypek and W. Ciesielski, Electroanalysis, 2012, 24, 1966–1972 CrossRef CAS.
- A. Nzila, J. Antimicrob. Chemother., 2006, 57, 1043–1054 CrossRef CAS PubMed.
- A. E. Radi, H. M. Nassef and M. I. Attallah, Anal. Methods, 2015, 7, 4159–4167 RSC.
- J. Dedik, M. Janovcova, H. Dejmkova, J. Barek and K. Peckova, in Sensing in Electroanalysis, ed. K. Kalcher, R. Metelka, I. Svancara and K. Vytras, University press centre, Pardubice, Czech Republic, 2011, vol. 6, pp. 129–138 Search PubMed.
- M. U. Ahmed, M. M. Hossain, M. Safavieh, Y. L. Wong, I. A. Rahman, M. Zourobd and E. Tamiya, Crit. Rev. Biotechnol., 2016, 36, 495–505 Search PubMed.
- H. M. Mohamed, TrAC, Trends Anal. Chem., 2016, 82, 1–11 CrossRef CAS.
- S. U. Ambalkar, R. L. Bakal, A. P. Dewani and A. V. Chandewar, Int. J. Drug Form. Res., 2013, 4, 26–44 Search PubMed.
- N. Thammasoontaree, P. Rattanarat, N. Ruecha, W. Siangproh, N. Rodthongkum and O. Chailapakul, Talanta, 2014, 123, 115–121 CrossRef CAS PubMed.
- S. Yao, J. Shiao and L. Nie, Talanta, 1987, 34, 977–982 CrossRef CAS PubMed.
- M. Smieja, Can. J. Infect. Dis., 1998, 9, 22–28 CrossRef CAS PubMed.
- P. Norouzi, B. Larijani, M. Ezoddin and M. R. Ganjali, Mater. Sci. Eng., C, 2008, 28, 87–93 CrossRef CAS.
- Z. Zhang, Y. Hu, H. Zhang and S. Yao, J. Colloid Interface Sci., 2010, 344, 158–164 CrossRef CAS PubMed.
- I. H. I. Habib, M. S. Rizk and Th. R. El-Aryan, Pharm. Chem. J., 2011, 44, 705–710 CrossRef CAS.
- K. R. Tan, A. J. Magill, M. E. Parise and P. M. Arguin, Am. J. Trop. Med. Hyg., 2011, 84, 517–531 CrossRef PubMed.
- E. van den Enden, Expert Opin. Pharmacother., 2009, 10, 435–451 CrossRef CAS PubMed.
- S. Chodosh, J. Tuck and D. Pizzuto, Scand. J. Infect. Dis., Suppl., 1988, 53, 22–28 CAS.
- J. Wang, T. Peng and M. S. Lin, Bioelectrochem. Bioenerg., 1986, 15, 147–156 CrossRef CAS.
- W. Oungpipat, P. Southwell-Keely and P. W. Alexander, Analyst, 1995, 120, 1559–1565 RSC.
- N. Wangfuengkanagul, W. Siangproh and O. Chailapakul, Talanta, 2004, 64, 1183–1188 CrossRef CAS PubMed.
- T. Charoenraks, S. Palaharn, K. Grudpan, W. Siangproh and O. Chailapakul, Talanta, 2004, 64, 1247–1252 CrossRef CAS PubMed.
- L. G. Shaidarova, A. V. Gedmina, I. A. Chelnokova and G. K. Budnikov, Pharm. Chem. J., 2008, 42, 545–549 CrossRef CAS.
- F. Conzuelo, M. Gamella, S. Campuzano, A. J. Reviejo and J. M. Pingarron, Anal. Chim. Acta, 2012, 737, 29–36 CrossRef CAS PubMed.
- B. Gurler, S. P. Ozkorucuklu and E. Kir, J. Pharm. Biomed. Anal., 2013, 84, 263–268 CrossRef CAS PubMed.
- A. F. Shoukry and S. S. Badawy, Microchem. J., 1987, 36, 107–112 CrossRef CAS.
- M. Pekli-Novak, A. Nagy and G. Nagy, Bioelectroanal., 1993, 2, 411–416 Search PubMed.
- M. Pekli-Novak, A. Nagy and G. Nagy, Magy. Kem. Foly., 1995, 101, 12–16 Search PubMed.
- X. X. Sun and H. Y. Aboul-Enein, Talanta, 2002, 58, 387–396 CrossRef CAS PubMed.
- A. H. Kamel, F. T. C. Moreira and M. G. F. Sales, Sens. Lett., 2011, 9, 1654–1660 CrossRef CAS.
- Y. M. Issa, H. M. Abdel-Fattah and N. B. Abdel-Moniem, Int. J. Electrochem. Sci., 2013, 8, 9578–9592 CAS.
- R. J. Maude, C. Nguon, A. M. Dondorp, L. J. White and N. J. White, Malar. J., 2014, 13, 380/1–380/8 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |