An efficient alkynylation of 4-thiazolidinone with terminal alkyne under C–H functionalisation†
Abstract
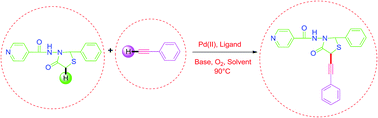
A method of palladium catalysed efficient alkynylation through the cross coupling reaction of terminal alkynes with the slightly more acidic C–H bonds of 4-thiazolidinone has been developed. This method allows the direct introduction of an ethynyl group into the 4-thiazolidinone moiety. Mild reaction conditions involving aerial oxidation and one pot synthesis make this reaction more efficient for the formation of sp3(C)–sp(C) bond.

 Please wait while we load your content...
Please wait while we load your content...