Lithium orthosilicate with halloysite as silicon source for high temperature CO2 capture
Abstract
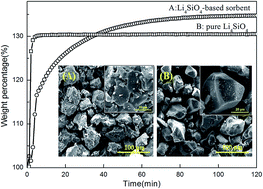
Lithium orthosilicate (Li4SiO4)-based sorbents were synthesized using a low cost and naturally available mineral resource (halloysite) as silicon source for high temperature CO2 capture. Halloysite nanotubes (HNTs) were acid-treated by 6 M HCl for 6 h to leach diaspore-like sheet to produce Si source (H6), which showed the best extraction effects. Pure Li4SiO4 (P-LS) and H6-Li4SiO4 (H6-LS) were thermally analyzed under a CO2 flux. X-ray diffraction (XRD), X-ray fluorescence (XRF), scanning electron microscopy (SEM), nitrogen adsorption, and thermogravimetric (TG) analysis were used to examine the character of the phase, morphology, and CO2 chemisorption properties of the samples. The Li4SiO4 sorbent was synthesized at 800 °C for 4 h (H6-LS800), and the metal impurities especially aluminum were doped into the structure of H6-LS800, resulting in the increased surface area. Thermal analysis indicated the maximum CO2 adsorption capacity of H6-LS800 reached up to 34.45%, with the temperature range from 350 to 720 °C, which had a better CO2 adsorption than P-LS. In addition, H6-LS800 sorbent exhibited excellent adsorption–desorption performance.

 Please wait while we load your content...
Please wait while we load your content...