Sensitive colorimetric detection of glucose and cholesterol by using Au@Ag core–shell nanoparticles†
Xuehong Zhanga,
Min Weib,
Bingjing Lva,
Yuanjian Liua,
Xu Liua and
Wei Wei*a
aLaboratory of Environmental Medicine Engineering, Ministry of Education, Jiangsu Province Hi-Tech Key Laboratory for Bio-medical Research, School of Chemistry and Chemical Engineering, Southeast University, Nanjing, 211189, China. E-mail: wei_wei98@163.com
bCollege of Food Science and Technology, Henan University of Technology, Zhengzhou, 450001, China
First published on 4th April 2016
Abstract
Glucose and cholesterol in human fluids are clinically important analytes and their sensitive detection is significant in diagnosis. In this study, Au@Ag core–shell nanoparticles (Au@Ag NPs) prepared by in situ growth of silver nanoparticles (AgNPs) on the surface of thiol-PEG-capped gold nanoparticles (AuNPs, 13 nm) have been successfully applied to fabricate a colorimetric biosensing platform for glucose and cholesterol. Au@Ag NPs showed an obvious absorbance peak at 375 nm due to the surface plasmon resonance (SPR) absorption of AgNPs. In the presence of cholesterol and glucose, H2O2 was produced under the catalytic action of their corresponding enzymes such as glucose oxidase (GOx) or cholesterol oxidase (ChOx), which etched the AgNPs shell of Au@Ag NPs. As a result, their characteristic absorbance at 375 nm decreased, accompanied with a perceptible color change from orange to red, which could be used to detect glucose and cholesterol by the naked eye. Under optimum conditions, the linear ranges for the detection of glucose and cholesterol by UV-vis spectroscopy were from 0.5 to 400 μM and 0.3 to 300 μM, respectively. The detection limit of the biosensors for glucose and cholesterol were 0.24 μM and 0.15 μM, respectively. The practical applications of this method in urine or human serum have been realized with satisfactory results. This work provides a simple and sensitive approach for glucose and cholesterol detection, which has broad application prospects in clinical diagnosis.
Introduction
The abnormal levels of clinical or biochemical indicators may be the sign of some diseases. To keep healthy and ensure quality of life, the control and management of the important human physiological indicators such as cholesterol and glucose levels is very important. Glucose is an important indicator for diabetes. Diabetes is one of the top ten killers to human health. According to the World Health Organization (WHO), more than 347 million people worldwide were afflicted by diabetes in the year of 2013 and the number is still on the increase. Blood glucose level is recommended to be tested daily for effective management of diabetes mellitus and reduction of associated complications. The presence of glucose in urine is an indication of worsening of diabetes, which is a more dangerous condition. Therefore, the detection of glucose in urine provides preliminary screening of the patients with high level diabetes (having renal glycosuria). Glucose detection is of great significance in the fields of food, chemistry, biology, and environmental protection.1–4 Cholesterol is an essential lipid for human body and a major component of intracellular membranes of mammalian cells. An excessive level of cholesterol in blood is related to heart disease, arteriosclerosis,5 hypertension,6 and diabetes,7 whereas a low level of cholesterol is associated with anemia, wasting syndrome,8 hypothyroidism,9 etc. As a result, highly sensitive cholesterol determination is also very important for medical diagnostics. A variety of methods have been developed for glucose and cholesterol assay, including fluorescence,10,11 electrochemistry,12,13 surface-enhanced Raman scattering (SERS),14 chemiluminescence,15,16 luminescence,17 and photoluminescence methods.18Noble metal nanoparticles (NPs), especially AuNPs and AgNPs, have drawn significant attention due to their unique tunable optical properties caused by SPR absorption ranging from visible to near-infrared region of the spectrum.19–21 In the past few decades, many monometallic NPs-based plasmonic detection methods were widely developed for the detection of various analytes, such as glucose, cholesterol,22 uric acid,23 DNA,24 metal ion,25 and enzymes.26 Compared to single-element nanoparticles, core–shell bimetallic NPs consisting of two different metallic elements exhibit improved physical and chemical properties (such as unique optical, electronic, and catalytic properties) due to versatile tunable plasmonic properties as well as extremely amplification of plasmon resonances induced by synergistic interplay between different metallic components.27,28 As is known to us all, AgNPs exhibit stronger and more sensitive SPR when compared to AuNPs.29 But the stabilization of AgNPs remains a challenge, because AgNPs suffer from easy oxidation and aggregation. However, it is fairly easy to prepare AuNPs with excellent controlled size and shape,30 good biocompatibility, excellent stability, broader SPR properties. To combine the advantages of AgNPs (plasmonic sensitivity) and AuNPs (chemical stability), Au@Ag NPs have been synthesized and widely used in many applications.31–34
Among those proposed methods for glucose and cholesterol sensing, colorimetric analysis that can be observed by the naked eyes without expensive or sophisticated instrumentation is becoming highly competitive biosensing technology. Most of the existing metal NPs-based colorimetric systems for H2O2 assay took advantage of the peroxidase-like catalytic activity of some nanomaterials,35–37 where these enzyme-like nanomaterials catalyzed H2O2 to oxidize corresponding substrate or chromogenic agents. These nanomaterials were just peroxidase mimics and additional chromogenic agents including 3,3′,5,5′-tetramethylbenzidine (TMB) or 2,2′-azino-bis(3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS2−) often be used. As for the detection of glucose/cholesterol, glucose oxidase (GOx)/cholesterol oxidase (ChOx) is usually immobilized onto NPs surface.38,39 He et al. have developed a new method for sensitive optical glucose sensing based on Ag/Au bimetallic nanoshells.40 Desirable results were achieved in this strategy. However, the conjugation of GOx on the surface of Ag/Au bimetallic nanoshells was involved in this method. The conjugation processes are laborious and time-consuming and often lead the aggregation of the NPs.
Herein, we propose a simple but sensitive method for detection of glucose and cholesterol by using Au@Ag NPs. The principle of the method is illustrated in Scheme 1. Au@Ag NPs were synthesized via in situ growth of AgNPs on the surface of thiol-PEG-capped AuNPs. In this process, we chose thiol-PEG-capped AuNPs instead of bare AuNPs. Because the attachment of thiol-PEG onto the AuNPs surface can greatly improve the stability of AuNPs in aqueous media. More importantly, the introduction of thiol-PEG on the surface of AuNPs impart high affinity to AgNPs through the formation of Ag–S colvent bond which can solve the problem of the repulsion between particles (AuNPs and AgNPs).41,42 As a result, the presence of p-aminophenol (p-AP) can effectively reduce silver ion to form AgNPs shell on the surface of thiol-PEG functionalized AuNPs even at mild temperature. AuNPs were wine red since its maximum absorbance wavelength was at 520 nm, while Au@Ag NPs were orange due to its maximum absorbance wavelength was at 375 nm. GOx/ChOx can specifically catalyze the oxidation of glucose/cholesterol to form H2O2 in the presence of oxygen.43 Then, AgNPs (Ag0) was oxidized to Ag+ by H2O2. As a result, the absorbance of AgNPs at 375 nm decreased accompanying with a slight blue-shift. A distinct color change from orange to light orange to light red and finally restored to wine red was observed when various concentration of glucose or cholesterol was present. Thus, the strategy that took advantage of the etching/dissolving of AgNPs shell from Au@Ag NPs by H2O2 was constructed for detection of glucose and cholesterol. This method displayed some advantages over the currently existing methods such as simple because no additional chromogenic agent was necessary, high sensitivity due to the high molar extinction coefficient of AgNPs,34,44 ease of operation, rapid/direct readout with naked-eyes, and applicable in complex samples.
 | ||
| Scheme 1 Schematic illustration of the formation of Au@Ag NPs and its application for the colorimetric detection of H2O2 and glucose/cholesterol. | ||
Experimental section
Reagents and materials
Glucose, fructose, sucrose, hydrogen peroxide (H2O2, 30%) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Silver nitrate (AgNO3), chloroauric acid tetrahydrate (HAuCl4·4H2O) and sodium citrate were purchased from Shanghai Reagent Company (Shanghai, China). Thiolated, methyl terminated ethylene glycol oligomers (thiol-PEG), glucose oxidase were purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO), p-aminophenol (p-AP) and various amino acids, monosodium phosphate (NaH2PO4), disodium phosphate (Na2HPO4), and cholesterol oxidase were purchased from Aladdin Industrial Inc. (Shanghai, China). Diethanolamine (DEA) was obtained from Shanghai Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). All other chemicals and reagents were of analytical reagent grade and used without further purification. In the experiment, all solutions were prepared with ultrapure water (18.2 MΩ cm, Barnstead, Thermo Scientific, USA). 20 mM of cholesterol stock solution was prepared by dissolving cholesterol in the mixture of isopropanol and Triton X-100 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). The cholesterol standard solutions were prepared by proper dilution of the stock cholesterol solution with PB (0.5 M, pH 7.4) then stored at 4 °C. DEA buffer solutions (600 mM DEA, 1 mM MgSO4, pH 9.8) were used for the deposition of AgNPs on the surface of AuNPs.
1, v/v). The cholesterol standard solutions were prepared by proper dilution of the stock cholesterol solution with PB (0.5 M, pH 7.4) then stored at 4 °C. DEA buffer solutions (600 mM DEA, 1 mM MgSO4, pH 9.8) were used for the deposition of AgNPs on the surface of AuNPs.
Apparatus and characterization
UV-vis absorption spectra were acquired by UV-vis spectrophotometer (Cary 100, Agilent, Singapore) from 800 to 300 nm. Transmission electron microscopy (TEM) measurements were obtained from a field emission transmission electron microscope (JEM-2010, Hitachi, Japan) under the accelerating voltage of 200 kV. To prepare samples for TEM characterization, a drop of sample solution was dispersed on a carbon-coated copper grid and the solvent was evaporated in air. Photographs were taken by virtue of a digital camera (DSC-W730, Sony, Japan).Synthesis of thiol-PEG-functionalized AuNPs
The AuNPs were synthesized according to the standard citrate reduction procedures with slight modifications. First of all, all glassware was thoroughly cleaned and soaked in freshly prepared aqua regia (HNO3/HCl = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). And it was rinsed with abundant amounts of ultrapure water and dried in oven. Secondly, HAuCl4 (1 mM, 50 mL) aqueous was preparation by dissolving HAuCl4·4H2O in ultrapure water and was poured into a round-bottom flask. Heat the flask under constant stirring until vigorous boil. Thirdly, when the solution was refluxing vigorously (1 drip per s), 5 mL of 38.8 mM sodium citrate solution was quickly added to the boiling solution. Reflux 15 min. During this period, the solution experienced a series of color changes from yellow to clear, to black, to purple and to wine red. Then turned off the heat and allowed solution to cool to room temperature. At last, AuNPs solution was stored in dark bottles at 4 °C for further use.
3). And it was rinsed with abundant amounts of ultrapure water and dried in oven. Secondly, HAuCl4 (1 mM, 50 mL) aqueous was preparation by dissolving HAuCl4·4H2O in ultrapure water and was poured into a round-bottom flask. Heat the flask under constant stirring until vigorous boil. Thirdly, when the solution was refluxing vigorously (1 drip per s), 5 mL of 38.8 mM sodium citrate solution was quickly added to the boiling solution. Reflux 15 min. During this period, the solution experienced a series of color changes from yellow to clear, to black, to purple and to wine red. Then turned off the heat and allowed solution to cool to room temperature. At last, AuNPs solution was stored in dark bottles at 4 °C for further use.
The attachment of thiol-PEG to AuNPs was prepared by rapidly adding 6 μL 25 mM thiol-PEG to the as-prepared 1000 μL of 13 nm AuNPs solution followed by a brief vortex mixing. After stirring for 2 h at room temperature, thiol-PEG was attached to AuNPs by the covalent bond between gold and sulfur. The final mixing solutions were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm and 4 °C for 25 min, and the supernatant was removed followed by resuspension in water. After repeating washing for twice, the thiol-PEG-capped AuNPs were resuspended in water, which can be stored at room temperature for months and are stable in aqueous solution with high salt concentration.
200 rpm and 4 °C for 25 min, and the supernatant was removed followed by resuspension in water. After repeating washing for twice, the thiol-PEG-capped AuNPs were resuspended in water, which can be stored at room temperature for months and are stable in aqueous solution with high salt concentration.
Fabrication of Au@Ag core–shell nanoparticles
Au@Ag NPs were prepared by in situ growth of AgNPs on the surface of AuNPs. In this procedure, 4 nM aqueous thiol-PEG functionalized AuNPs and 1 mM AgNO3 solution were first mixed in DEA buffer solutions (600 mM DEA, 1 mM MgSO4, pH 9.8), followed by addition of 125 μM p-AP to the above solution. After reaction for 40 min at room temperature, the final Au@Ag NPs were characterized or used for following experiments.Glucose/cholesterol detection
A total volume of 150 μL of sample mixtures containing 50 μL of GOx (3.33 mg mL−1)/ChOx (1.5 mg mL−1) and 100 μL of glucose/cholesterol at various concentrations were incubated at 37 °C for 30 min. 350 μL of as-prepared Au@Ag NPs was subsequently added to the sample mixtures. After reaction at room temperature for 30 min, the absorption spectra of final mixture were recorded with a UV-vis spectrophotometer.Real sample detection
The urine and serum sample were diluted 20 times before use, respectively. Afterwards, 50 μL of GOx (3.33 mg mL−1)/ChOx (1.5 mg mL−1) and 50 μL of diluted samples spiked with 50 μL of varying concentration of glucose/cholesterol were incubated at 37 °C for 30 min. Then 350 μL of as-prepared Au@Ag NPs dispersion was subsequently added to the prepared sample mixtures. After incubation at room temperature for 40 min, the absorbance of the above solution was measured at room temperature.Results and discussion
Feasibility and characterization of the proposed strategy for H2O2 and glucose/cholesterol sensing
A series of experiments were conducted to demonstrate feasibility of the proposed method. Fig. 1 is the UV-vis absorption spectra of detection solution. The thiol-PEG stabilized AuNPs were dispersed in DEA buffer and the color of the dispersion remained wine red, which indicated the good stability of thiol-PEG-capped AuNPs. The maximum absorbance wavelength of AuNPs was at 520 nm (Fig. 1, curve a). No obvious absorbance change occurred when AgNO3 (Fig. 1, curve b) or p-AP (Fig. 1, curve c) was added to the AuNPs solutions. After addition of p-AP and AgNO3 to thiol-PEG-stabilized AuNPs dispersion, the color of suspension changed from wine red to orange and a new strong absorption peak at around 375 nm appeared owing to the in situ growth of AgNPs shell on the surface of AuNPs (Fig. 1, curve d). SPR absorption peak of AuNPs underwent a blue-shift also indicated the formation of AgNPs coating around AuNPs.45,46 In the presence of H2O2, the SPR absorbance at 375 nm decreased obviously, the color changed from orange to wine red, which proved that AgNPs was etched through the effectively oxidation of Ag0 to Ag+ by H2O2 (Fig. 1, curve e). As a consequence, we concluded that AgNPs was etched by H2O2 on the basis of previous reports.38,47,48 The photographs of the corresponding solution were shown in the inset of Fig. 1.The morphology of the thiol-PEG functionalized AuNPs was also characterized by TEM. The uniform and spherical AuNPs were well-dispersed with an average diameter of 13 nm (Fig. 2A). The successful fabrication of Au@Ag core–shell nanoparticles was also demonstrated by TEM, it is noteworthy that the average size of the as-synthesized nanostructures was significantly larger than that of AuNPs (Fig. 2B). The AuNPs core was completely enclosed with a layer of AgNPs. After addition of H2O2 to the Au@Ag core–shell nanoparticles solution, the corresponding TEM image showed that the size of gold core remained almost constant, while the thickness of the deposited silver on the AuNPs significantly decreased (Fig. 2C), the above results further proved that AgNPs was etched by H2O2.
 | ||
| Fig. 2 TEM images of (A) AuNPs (13 nm), Au@Ag core–shell nanoparticles (B) before and (C) after the addition of H2O2. | ||
Optimization of experimental conditions for colorimetric detection
The detection sensitivity for glucose depended on the function of H2O2 on the Au@Ag NPs. To achieve the best assay performance, several important experimental parameters including deposition time for AgNPs, p-AP concentration, AgNO3 concentration and deposition temperature were investigated in this work. As can be seen from Fig. S1A,† the reduction of Ag+ by p-AP in the presence of AuNPs was completed in 40 min. It was observed that the signal change (A0 − A) (A0 and A are absorbance at 375 nm in the absence and the presence of 250 μM H2O2, respectively) increased notably with the increasing concentration of p-AP and AgNO3 and tended to be constant when the concentration of p-AP and AgNO3 reached 125 μM (Fig. S1B†) and 1 mM (Fig. S1C†), respectively. As a result, 125 μM p-AP and 1 mM AgNO3 was adopted in the detection experiments. The influences of deposition temperature were investigated in Fig. S1D.† It was noteworthy that good response was obtained at 10–40 °C. Considering of the convenience of the practical operation, the room temperature (25 °C) was chosen for subsequent experiments.Glucose assay and cholesterol assay performance
Under the optimum conditions, the detection capability of the proposed strategy for H2O2, glucose and cholesterol sensing is evaluated. As is illustrated in Fig. S2,† the color of the solution distinctly changed from orange to wine red with the increase of H2O2 concentrations (inset in Fig. S2A†). Accordingly, the UV-vis absorption spectra of the detection solution showed that the absorbance decreased gradually with the addition of increasing concentration of H2O2. The absorbance versus the concentration of H2O2 was plotted in Fig. S2B.† There is a good linear response (y = 1.0808 − 0.0025x, R2 = 0.9986) between absorbance and the concentration of H2O2 in the range from 0.5 to 250 μM (inset in Fig. S2B†), with a detection limit of 0.11 μM (S/N = 3).Glucose oxidase (GOx) and cholesterol oxidase (ChOx) are commonly used in glucose and cholesterol sensing, respectively. GOx/ChOx can specifically catalyze the oxidation of glucose/cholesterol in the presence of oxygen to produce H2O2. The enzyme-catalyzed reaction could be described by eqn (1) and (2), respectively.
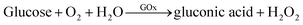 | (1) |
 | (2) |
Consequently, the produced H2O2 can act as a mediator to construct oxidase-based sensors. As is depicted in Fig. 3A and C, a gradual decrease of absorbance could be observed as the concentration of glucose/cholesterol increased. The response curve of absorbance versus glucose/cholesterol concentration is illustrated in Fig. 3B and D. The linear range for glucose and cholesterol was from 0.5 to 400 μM (inset in Fig. 3B) and 0.3 to 300 μM (inset in Fig. 3D), respectively, with a linear coefficient R2 = 0.9985 and R2 = 0.9988. The linear regression equation for glucose and cholesterol were as follows: y = 1.0862 − 0.0017x and y = 1.0696 − 0.0022x, respectively. The detection limit for glucose and cholesterol was as low as 0.24 μM and 0.15 μM (S/N = 3), respectively. The color change towards different concentration of glucose and cholesterol were shown in Fig. 3A and C (inset), respectively.
To get a clear perspective of the performance of the proposed method, a comparison between the present glucose/cholesterol sensing system with some of the reported methods was shown in Table S1.† The detection limit of this method was better or comparable to most previously reported methods. And the proposed approach exhibited higher sensitivity, a wider linear range for glucose/cholesterol detection. In addition, the semi-quantitative and qualitative detection of glucose/cholesterol by the proposed method can be realized by naked eyes without the need of sophisticated instruments. No additional chromogenic agent, laborious and time-consuming enzyme conjugation processes were necessary. The detection processes were conducted at room temperature and ambient conditions. Thus, the operation of experiments was relatively simple and convenient.
The reproducibility of the assay were determined by calculating intra/inter batch variability (n = 5). Intra-assay and inter-assay variability were calculated by conducting five times measurements of 200 μM of glucose/cholesterol with the same batch of Au@Ag NPs and detecting 200 μM of glucose/cholesterol using five batches of Au@Ag NPs, respectively. The intra-assay relative standard deviation (RSD) for glucose and cholesterol were 1.2% and 3.6%, respectively. Inter-assay RSD for them were 2.0% and 5.4%, respectively. These results indicated that the proposed sensor exhibited excellent reproducibility and precision.
The selectivity for glucose and cholesterol were evaluated by testing some potential interfering substances such as fructose, sucrose, lactose, galactose, glycine, methionine, arginine, K+, Na+. As is shown in Fig. 4A and B, the absorbance intensity at 375 nm decreased dramatically in the presence of 300 μM glucose and cholesterol, respectively, while 600 μM or 1 mM of these interferential species exerted no notable influence on the absorbance of detection solution. As a consequence, this assay exhibited high selectivity in the determination of glucose/cholesterol.
Real sample detection
The practicability of the sensors for glucose/cholesterol detection was investigated in a healthy person's urine and serum. The recovery measurements were first carried out. The results were listed in Table 1. The concentration of glucose in intact urine sample was 0.580 mM, which is in good agreement with the fact that the concentration of glucose in normal person is below 1 mM.49 1, 5, 10 and 30 mM of glucose were added in this healthy urine to evaluate the precision and accuracy of the method. The recoveries ranged from 97.2% to 104.6% and RSD were less than 3.83%, which indicating the proposed method had good accuracy and high precision for glucose assay. 1.544 mM of cholesterol was detected by the proposed method, which was reasonable for the concentration of free cholesterol varied from 1.29 mM to 2.07 mM in human serum.50 1, 5, 10 and 30 mM of cholesterol were respectively added in human serum to evaluate the precision and accuracy of the sensor. All the data were summarized in Table 2, the recovery varied from 93.5% to 104.3%, and the RSD were less than 5.88%. Thus, the results demonstrate that the developed sensor is applicable to the detection of glucose/cholesterol in complicated samples with favorable accuracy and precision.| Analytes | Spiked (mM) | Found (mM) | Recovery (%) | RSD (%) (n = 3) |
|---|---|---|---|---|
| Glucose | 0 | 0.580 | — | 0.84 |
| 1 | 1.552 | 97.2 | 0.51 | |
| 5 | 5.530 | 99.0 | 1.02 | |
| 10 | 11.041 | 104.6 | 2.66 | |
| 30 | 31.037 | 101.5 | 3.83 | |
| Cholesterol | 0 | 1.544 | — | 0.90 |
| 1 | 2.610 | 106.6 | 0.43 | |
| 5 | 6.320 | 95.6 | 2.69 | |
| 10 | 11.715 | 101.7 | 1.88 | |
| 30 | 31.283 | 99.1 | 5.88 |
| Sample | This work (mM) | RSD (%) (n = 3) | Glucometer (mM) | RSD (%) (n = 3) |
|---|---|---|---|---|
| 1 | 4.83 | 2.69 | 4.70 | 4.26 |
| 2 | 7.30 | 4.46 | 7.53 | 3.34 |
| 3 | 8.79 | 4.82 | 8.97 | 1.70 |
| 4 | 10.36 | 3.09 | 10.23 | 2.46 |
To further investigate the application possibility of the Au@Ag NPs based colorimetric assay for the glucose determination in clinical human serum samples, the glucose level in human serum samples from one healthy and three diabetes patients were monitored. The obtained experimental results were comparable to those measured by the glucometer (Table 2), indicating the proposed glucose sensor can be utilized for practical sample testing.
Conclusions
To sum up, we have successfully developed Au@Ag NPs based sensor for the quantitative detection of glucose and cholesterol based on the etching effect of H2O2 produced fromenzymatic oxidation of analytes on AgNPs shell. We developed a single-step approach for the growth of AgNPs on the surface of AuNPs. This approach exhibited a high sensitivity and favorable selectivity towards glucose/cholesterol over other interfering substances due to the high extinction coefficient of AgNPs and the specificity of enzymatic catalysis. The methods can be applied to detect analytes in complex samples with satisfactory results. The quasi-quantification of glucose and cholesterol could be observed by naked eyes. The method is simple compared with the traditional TMB-based colorimetric systems because Au@Ag NPs acts as both reducing agent and color indicator, therefore, no extra chromogenic substrate was needed. The assay platform is also can be applied for selective detection of other biological substrates which could be oxidized to produce H2O2 with the aid of corresponding enzyme (e.g., choline, xanthine, and lactic acid). Hence, the proposed strategy provides a new facile approach to develop simple, sensitive and effective sensors for biochemical and clinical applications.Acknowledgements
The project was supported by National Natural Science Foundation of China (Grant No. 21475020 and 21375014) and by the priority academic program development of Jiangsu higher education institutions.Notes and references
- J. L. Liu, L. L. Lu, A. Q. Li, J. Tang, S. G. Wang, S. Y. Xu and L. Y. Wang, Biosens. Bioelectron., 2015, 68, 204–209 CrossRef CAS PubMed.
- G. Rocchitta, O. Secchi, M. D. Alvau, D. Farina, G. Bazzu, G. Calia, R. Migheli, M. S. Desole, R. D. O'Neill and P. A. Serra, Anal. Chem., 2013, 85, 10282–10288 CrossRef CAS PubMed.
- L. A. Terry, S. F. White and L. J. Tigwell, J. Agric. Food Chem., 2005, 53, 1309–1316 CrossRef CAS PubMed.
- Y. Xiang and Y. Lu, Anal. Chem., 2012, 84, 1975–1980 CrossRef CAS PubMed.
- P. W. F. Wilson, R. B. D'Agostino, D. Levy, A. M. Belanger, H. Silbershatz and W. B. Kannel, Circulation, 1998, 97, 1837–1847 CrossRef CAS PubMed.
- S. K. Arya, M. Datta and B. D. Malhotra, Biosens. Bioelectron., 2008, 23, 1083–1100 CrossRef CAS PubMed.
- J. Kumar and S. F. D'Souza, BARC Newsl., 2012, 38, 34–38 Search PubMed.
- S. Soylemez, S. O. Hacioglu, M. Kesik, H. Unay, A. Cirpan and L. Toppare, ACS Appl. Mater. Interfaces, 2014, 6, 18290–18300 CAS.
- M. M. Rahman, PLoS One, 2014, 9, e100327 Search PubMed.
- Z. G. Mao, Z. H. Qing, T. P. Qing, F. Z. Xu, L. Wen, X. X. He, D. G. He, H. Shi and K. M. Wang, Anal. Chem., 2015, 87, 7454–7460 CrossRef CAS PubMed.
- A. Mondal and N. R. Jana, Chem. Commun., 2012, 48, 7316–7318 RSC.
- C. Y. Guo, H. H. Huo, X. Han, C. L. Xu and H. L. Li, Anal. Chem., 2014, 86, 876–883 CrossRef CAS PubMed.
- J. Ji, Z. Z. Zhou, X. L. Zhao, J. D. Sun and X. L. Sun, Biosens. Bioelectron., 2015, 66, 590–595 CrossRef CAS PubMed.
- X. S. Bi, X. Z. Du, J. J. Jiang and X. Huang, Anal. Chem., 2015, 87, 2016–2021 CrossRef CAS PubMed.
- L. Hong, A. L. Liu, G. W. Li, W. Chen and X. H. Lin, Biosens. Bioelectron., 2013, 43, 1–5 CrossRef CAS PubMed.
- F. Q. Luo, Y. L. Lin, L. Y. Zheng, X. M. Lin and Y. W. Chi, ACS Appl. Mater. Interfaces, 2015, 7, 11322–11329 CAS.
- Y. C. Shiang, C. C. Huang and H. T. Chang, Chem. Commun., 2009, 23, 3437–3439 RSC.
- Y. H. Yi, J. H. Deng, Y. Y. Zhang, H. T. Li and S. Z. Yao, Chem. Commun., 2013, 49, 612–614 RSC.
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- W. Lewandowski, M. Fruhnert, J. Mieczkowski, C. Rockstuhl and E. Górecka, Nat. Commun., 2015, 6, 6590 CrossRef PubMed.
- M. Rycenga, C. M. Cobley, J. Zeng, W. Y. Li, C. H. Moran, Q. Zhang, D. Qin and Y. N. Xia, Chem. Rev., 2011, 111, 3669–3712 CrossRef CAS PubMed.
- V. Raj, T. Johnson and K. Joseph, Biosens. Bioelectron., 2014, 60, 191–194 CrossRef CAS PubMed.
- R. S. Dey and C. R. Raj, ACS Appl. Mater. Interfaces, 2013, 5, 4791–4798 CAS.
- W. Xu, X. J. Xue, T. H. Li, H. Q. Zeng and X. G. Liu, Angew. Chem., Int. Ed., 2009, 48, 6849–6852 CrossRef CAS PubMed.
- J. S. Lee, M. S. Han and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 46, 4093–4096 CrossRef CAS PubMed.
- H. Wei, C. G. Chen, B. Y. Han and E. K. Wang, Anal. Chem., 2008, 80, 7051–7055 CrossRef CAS PubMed.
- S. K. Cha, J. H. Mun, T. Chang, S. Y. Kim, J. Y. Kim, H. M. Jin, J. Y. Lee, J. Shin, K. H. Kim and S. O. Kim, ACS Nano, 2015, 5, 5536–5543 CrossRef PubMed.
- D. Ferrer, A. Torres-Castro, X. Gao, S. Sepúlveda-Guzmán, U. Ortiz-Méndez and M. José-Yacamán, Nano Lett., 2007, 7, 1701–1705 CrossRef CAS PubMed.
- A. Jakab, C. Rosman, Y. Khalavka, J. Becker, A. Trügler, U. Hohenester and C. Sönnichsen, ACS Nano, 2011, 5, 6880–6885 CrossRef CAS PubMed.
- J. Zhang, H. Liu, Z. Wang and N. Ming, Adv. Funct. Mater., 2007, 17, 3295–3303 CrossRef CAS.
- A. K. Samal, L. Polavarapu, S. Rodal-Cedeira, L. M. Liz-marza and I. Pastoriza-Santos, Langmuir, 2013, 29, 15076–15082 CrossRef CAS PubMed.
- C. I. Wang, W. T. Chen and H. T. Chang, Anal. Chem., 2012, 84, 9706–9712 CrossRef CAS PubMed.
- P. Y. Yuan, R. Z. Ma, N. Y. Gao, M. Garai and Q. H. Xu, Nanoscale, 2015, 7, 10233–10239 RSC.
- C. H. Zhou, J. Y. Zhao, D. W. Pang and Z. L. Zhang, Anal. Chem., 2014, 86, 2752–2759 CrossRef CAS PubMed.
- L. Han, L. X. Zeng, M. D. Wei, C. M. Li and A. H. Liu, Nanoscale, 2015, 7, 11678–11685 RSC.
- X. Jiang, C. J. Sun, Y. Guo, G. J. Nie and L. Xu, Biosens. Bioelectron., 2015, 64, 165–170 CrossRef CAS PubMed.
- L. Su, J. Feng, X. M. Zhou, C. L. Ren, H. H. Li and X. G. Chen, Anal. Chem., 2012, 84, 5753–5758 CrossRef CAS PubMed.
- J. Huan, Q. Liu, A. R. Fei, J. Qian, X. Y. Dong, B. J. Qiu, H. P. Mao and K. Wang, Biosens. Bioelectron., 2015, 73, 221–227 CrossRef CAS PubMed.
- C. Lu, X. J. Liu, Y. F. Li, F. Yu, L. H. Tang, Y. J. Hu and Y. B. Ying, ACS Appl. Mater. Interfaces, 2015, 7, 15395–15402 CAS.
- H. L. He, X. L. Xu, H. X. Wu and Y. D. Jin, Adv. Mater., 2012, 24, 1736–1740 CrossRef CAS PubMed.
- J. K. Yang, H. Kang, H. Lee, A. Jo, S. Jeong, S. J. Jeon, H. I. Kim, H. Y. Lee, D. H. Jeong, J. H. Kim and Y. S. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 12541–12549 CAS.
- J. Y. Gui, D. A. Stern, D. G. Frank, F. Lu, D. C. Zapien and A. T. Hubbard, Langmuir, 1991, 7, 955–963 CrossRef CAS.
- T. Wen, F. Qu, N. B. Li and H. Q. Luo, Anal. Chim. Acta, 2012, 749, 56–62 CrossRef CAS PubMed.
- J. S. Lee, A. K. R. Lytton-Jean, S. J. Hurst and C. A. Mirkin, Nano Lett., 2007, 7, 2112–2115 CrossRef CAS PubMed.
- L. M. Liz-Marzan, Langmuir, 2006, 22, 32–41 CrossRef CAS PubMed.
- L. Rodríguez-lorenzo, R. de la Rica, R. A. Álvarez-puebla, L. M. Liz-Marzán and M. M. Stevens, Nat. Mater., 2012, 11, 10–13 CrossRef PubMed.
- H. L. He, X. L. Xu, H. X. Wu, Y. J. Zhai and Y. D. Jin, Anal. Chem., 2013, 85, 4546–4553 CrossRef CAS PubMed.
- Q. Zhang, C. M. Cobley, J. Zeng, L. P. Wen, J. Y. Chen and Y. N. Xia, J. Phys. Chem. C, 2010, 114, 6396–6400 CAS.
- D. Sechi, B. Greer, J. Johnson and N. Hashemi, Anal. Chem., 2013, 85, 10733–10737 CrossRef CAS PubMed.
- J. Motonaka and L. R. Faulkner, Anal. Chem., 1993, 65, 3258 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04976a |
| This journal is © The Royal Society of Chemistry 2016 |



