A direct carbon fuel cell with a CuO–ZnO–SDC composite anode
Wenbin Hao and
Yongli Mi*
Department of Chemical and Biomolecular Engineering, The Hong Kong University of Science and Technology, Room 4574, Academic Building, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: keymix@ust.hk; Fax: +852-23580054; Tel: +852-23587127
First published on 6th May 2016
Abstract
A direct carbon fuel cell with a copper oxide–zinc oxide–Sm0.2Ce0.8O2−x (CuO–ZnO–SDC) composite anode was demonstrated. Nano-sized SDC and CuO–ZnO were synthesized by utilizing a co-precipitation process and a solid state reaction, respectively. As-prepared materials were characterized by various techniques, and then used to fabricate single cells by a co-press and co-sintering method. Finally, a button cell was tested with a mixture of carbon black and ternary (Li, Na, and K) eutectic carbonates as the anode fuel. The inert environment of the anode compartment was protected by pure N2 or a mixture of N2 & CO2. A maximum power density of 130 mW cm−2 was achieved using the mixture of N2 & CO2 as the anode gas at 700 °C. The results indicate that CuO–ZnO can be used as a nickel-free anode material for direct carbon fuel cells. Also, the modified anode gas of a N2 & CO2 mixture can enhance the performance of direct carbon fuel cells with a CuO–ZnO–SDC composite anode.
1 Introduction
The direct carbon fuel cell (DCFC) is a promising technology that directly consumes solid carbons to produce electricity via a pure electrochemical process.1 The overall reaction of a DCFC and its cathodic reaction are quite simple, as shown in reactions (1) and (2). However, the anodic reaction mechanism of DCFC is very complex. Hypothetical mechanisms are presented in reactions (3)–(8), according to different types of cell configurations.2The overall reaction of DCFC is given by reaction (1):
| C(s) + O2(g) = CO2(g) | (1) |
The cathodic reaction is given by reaction (2):
| O2(g) + 4e− = 2O2− | (2) |
The anodic reactions in the solid anode configuration are given by reactions (3)–(5):
| C(s) + 2O2− = CO2(g) + 4e− | (3) |
| C(s) + O2− = CO(g) + 2e− | (4) |
| CO(g) + O2− = CO2(g) + 2e− | (5) |
The anodic reactions in the molten carbonate configuration are given by reactions (6)–(8):
| C(s) + 2CO32− = 3CO2(g) + 4e− | (6) |
| C(s) + CO32− = CO(g) + CO2(g) + 2e− | (7) |
| CO(g) + CO32− = 2CO2(g) + 2e− | (8) |
In high temperature DCFCs, the Boudouard reaction, as shown in reaction (9), will also affect the reactions of carbon fuels in the anode.3
| C(s) + CO2(g) = 2CO(g) | (9) |
At present, this technology still faces many challenges, including the poor activation and short-term stability of the anode, the high costs of maintaining the operation, and the insufficient ways of scaling it up for practical applications.3,4 An ideal anode of DCFCs should be chemically stable enough to reduce the cost of long-term operation, mechanically strong enough to scale up the size of DCFC for the demand of practical applications, and as well as having reasonable fabrication cost. Thus, the key issue to address the challenges is to improve the anode performance.
So far, two methods have been developed to tackle this issue, namely the modification of anode structure and the exploration of new anode material. In the modification of anode structure, a wetting intermediate is introduced between the anode and carbon fuels, such as molten salts and melting redox pairs of metals/metal oxides.5–10 In the exploration of new anodes, several materials have been widely used in past DCFC studies. The most commonly used are nickel-based anodes, such as Ni–yttria stabilized zirconia (Ni–YSZ), Ni–samaria doped ceria (Ni–SDC) or Ni–gallium doped ceria (Ni–GDC), but they have some flaws including low sulfur tolerance and anode degradation due to the poisoning effect of sulfur on nickel catalysts.3,4,11 The flaws limit the fuel feedstock of DCFCs, especially for the direct utilization of fossil fuels, such as coal and natural gas.12,13 Some nickel-free materials with perovskite structure have also been developed as the anodes of DCFCs, such as La0.6Sr0.4Co0.2Fe0.8O3 (LSCF) and La0.3Sr0.7Co0.07Ti0.93O3 (LSCT).14–16 These materials that contain lanthanum – a precious rare earth metal element – were first used in the studies of solid oxide fuel cells (SOFCs). Silver was also added to these materials to form composites, in order to enhance the anode performance of DCFCs.17 The use of precious metals in the DCFC anode makes this type of anodes very costly for practical applications. Moreover, the procedures to process these materials are complex, and some of them require unique techniques and extreme conditions, such as tape casting and high sintering temperature up to 1500 °C.18 However, the above mentioned flaws can be avoided by developing cost-effective nickel-free anodes for DCFCs.
As described in this paper, a DCFC with a nickel-free anode, composed of a CuO–ZnO–SDC composite, was fabricated with a simpler process, and investigated at an intermediate temperature range. An excellent cell performance was achieved using such a composite as the anode at 700 °C. In addition, the influence of the inert gases in the anode compartment was studied as well.
2 Experimental
2.1 Preparation of cell materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to form a homogeneous solution. A proper amount of NaHCO3 (Sigma-Aldrich) solution (1.0 mol L−1) was added to the solution at a slow rate (10 mL min−1), under vigorous stirring. The molar ratio of cations (Ce3+, Sm3+) and carbonate ion was 1
1) to form a homogeneous solution. A proper amount of NaHCO3 (Sigma-Aldrich) solution (1.0 mol L−1) was added to the solution at a slow rate (10 mL min−1), under vigorous stirring. The molar ratio of cations (Ce3+, Sm3+) and carbonate ion was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. A suspension of ceria/samaria carbonate was acquired by a chemical co-precipitation process. The suspension was filtered by the suction filtration method. The acquired white precipitate was first washed with double deionized water for three times to remove the carbonate ion, and then washed with ethanol for three times to remove the water. The washing solution was examined with a CaCl2 solution (0.1 mol L−1) to ensure the complete removal of carbonate ions. The washed pale yellow precipitate was dried at 80 °C in an oven overnight. Then, the dried solid was sintered at 800 °C in a muffle furnace for 2 hours. Finally, the obtained yellow powder was used as the electrolyte material.
2. A suspension of ceria/samaria carbonate was acquired by a chemical co-precipitation process. The suspension was filtered by the suction filtration method. The acquired white precipitate was first washed with double deionized water for three times to remove the carbonate ion, and then washed with ethanol for three times to remove the water. The washing solution was examined with a CaCl2 solution (0.1 mol L−1) to ensure the complete removal of carbonate ions. The washed pale yellow precipitate was dried at 80 °C in an oven overnight. Then, the dried solid was sintered at 800 °C in a muffle furnace for 2 hours. Finally, the obtained yellow powder was used as the electrolyte material.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn molar ratio of 1
Zn molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) of CuCO3·Cu(OH)2·H2O (Nacalai Tesque) and ZnNO3·6H2O (Sigma-Aldrich) were mixed, and ball-milled at 400 rpm in a planet type machine (QM-3SP04, Nanjing University) for 4 hours. Then the mixture was pre-calcined at 700 °C for 3 hours to remove the carbonates and hydroxides. After ball-milling with the synthesized SDC in a 1
4) of CuCO3·Cu(OH)2·H2O (Nacalai Tesque) and ZnNO3·6H2O (Sigma-Aldrich) were mixed, and ball-milled at 400 rpm in a planet type machine (QM-3SP04, Nanjing University) for 4 hours. Then the mixture was pre-calcined at 700 °C for 3 hours to remove the carbonates and hydroxides. After ball-milling with the synthesized SDC in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volumetric ratio, the mixture was sintered at 800 °C in a muffle furnace for 4 hours. The acquired grey powder was used as the anode material.
1 volumetric ratio, the mixture was sintered at 800 °C in a muffle furnace for 4 hours. The acquired grey powder was used as the anode material.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni molar ratio of 1
Ni molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 700 °C for 3 hours.21 The acquired black powder was ball-milled with the synthesized SDC in a 1
1) at 700 °C for 3 hours.21 The acquired black powder was ball-milled with the synthesized SDC in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volumetric ratio. Finally, the acquired light black powder was used as the cathode material. The carbon fuel was obtained by ball-milling the mixture of carbon black and ternary (Li, Na, and K, molar ratio of 3
1 volumetric ratio. Finally, the acquired light black powder was used as the cathode material. The carbon fuel was obtained by ball-milling the mixture of carbon black and ternary (Li, Na, and K, molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) eutectic carbonates (melting point ∼ 450 °C) in a mass ratio of 1
4) eutectic carbonates (melting point ∼ 450 °C) in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 3000 rpm for 5 hours in a rolling machine.
1 at 3000 rpm for 5 hours in a rolling machine.2.2 Characterization of electrolyte and anode materials
The synthesized electrolyte and anode materials were characterized by X-ray power diffraction (XRD), scanning electron microscope (SEM) and transmission electron microscope (TEM). Crystal structures of SDC and CZO were examined via Philips PW1830 powder X-ray diffraction spectrometer equipped with a CuKα radiation source (wavelength of 1.540562 Å) and graphite monochromator. The scan range was from 20° to 80°. The acquired data were analyzed by MDI Jade 6.0. The SEM images were obtained to investigate the morphology information of SDC and CZO via JEOL 7100F. The high-resolution TEM images were captured to further investigate the microstructure information of SDC and CZO via JEOL 2010 and 2010F.2.3 Cell fabrication
A button cell was prepared for the cell performance test via a co-press and co-sintering process. Fixed amounts of anode, electrolyte, and cathode powders were sieved through a # 300 mesh and loaded in a round mold layer by layer. Then, the powders were co-pressed under 15 t load (∼300 MPa) at 80 °C in a hot press machine for 10 minutes. The acquired three-layered disk was co-sintered at 800 °C in a muffle furnace for 3 hours to form the button cell. The diameter of the button cell was 25 mm with a thickness of ∼0.5 mm. Finally, silver paste was printed on the anode and cathode, and then calcined at 550 °C for 1 hour to form the current collectors. The active area of the button cell was ∼1.25 cm2. The morphology information of the button cell cross-section was investigated via JEOL 6700F, equipped with back scattering electron (BSE) analyzer and energy-dispersive X-ray spectrometer (EDX).2.4 Cell performance test
A button cell was tested and the schematic of the experimental setup is shown in Fig. 1. In the performance test, 1.0 g carbon fuel was fed into the device. Pure N2 or a mixture of N2 & CO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volumetric ratio) were purged into the anode compartment at a total flow rate of 50 mL min−1. Compressed air was used as the oxidant and supplied into the cathode compartment at a flow rate of 100 mL min−1. Then, the cell was heated up and operated at 500–700 °C. The electrochemical properties, such as open circuit voltage (OCV), electrochemical impedance spectroscopy (EIS), I–V curves, and the fuel utilization in the cell were investigated via a electrochemical working station (CHI 608D & 680).
1 in volumetric ratio) were purged into the anode compartment at a total flow rate of 50 mL min−1. Compressed air was used as the oxidant and supplied into the cathode compartment at a flow rate of 100 mL min−1. Then, the cell was heated up and operated at 500–700 °C. The electrochemical properties, such as open circuit voltage (OCV), electrochemical impedance spectroscopy (EIS), I–V curves, and the fuel utilization in the cell were investigated via a electrochemical working station (CHI 608D & 680).
3 Results and discussion
3.1 Microstructure analysis of electrolyte and anode materials
The XRD patterns of the synthesized SDC, CZO, and CuO–ZnO–SDC composite are shown in Fig. 2a–c, respectively. In Fig. 2a, all the peaks are in accordance with the peaks of CeO2 (PDF # 81-0792), which has a cubic fluorite-type structure. The calculated lattice constant was 5.434 Å which is larger than the lattice constant of pure CeO2 (5.411 Å). The result is in agreement with Vegard's rule.22 As the ionic radius of Sm3+ is larger than that of Ce4+, the doping of Sm3+ would increase the lattice constant of the CeO2 crystal. This confirms that Sm3+ ions have been doped into the crystal lattice of CeO2. Fig. 2b gives the XRD patterns of the CZO. The peaks in the XRD patterns were examined to only belong to CuO and ZnO. The content of Cu and Zn in the CZO were calculated, based on the peak intensity of CuO and ZnO by MDI Jade 6.0. The result was consistent with the designed stoichiometry of CZO. In Fig. 2c, the XRD patterns of the composite suggest solely present of CZO and SDC. This indicates that the CuO–ZnO–SDC composite was well prepared.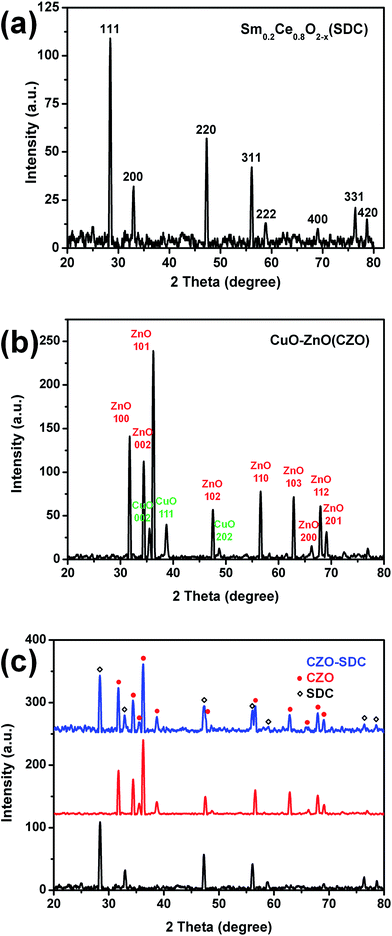 | ||
| Fig. 2 The XRD patterns of synthesized electrolyte and anode materials: (a) SDC, (b) CZO, and (c) CuO–ZnO–SDC composite with the XRD patterns of SDC and CZO as controls. | ||
The SEM images of the synthesized SDC and CZO are displayed in Fig. 3a and b, respectively. It can be seen clearly in Fig. 3a that the synthesized SDC showed polyhedral shape particles in the morphology investigation. The size distribution was narrow with an average particle size of ∼100 nm. In Fig. 3b, the similar-shaped CZO particles were displayed with an average particle size of ∼200 nm. It can also be observed that the SDC provides more uniform distribution in terms of particle size than does the CZO.
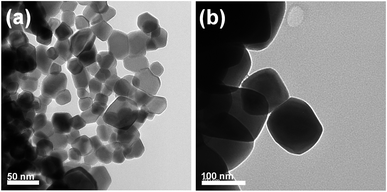
TEM was employed to further investigate the micro-structure of the SDC and CZO. The TEM images of SDC and CZO that were captured are shown in Fig. 4a and b. The TEM result agrees with the SEM result.
The SEM image of the button cell cross-section is shown in Fig. 5, together with the image of BSE mode. From the SEM and BSE images, it can be seen clearly that three functional layers were well formed via the co-press and co-sintering process. The porous anode and cathode was separated by the dense electrolyte, which indicates the desired micro-structure was obtained. The thickness of each layer was measured according to the scale bar (cathode 65.6 μm, electrolyte 78.4 μm, and anode ∼300 μm).
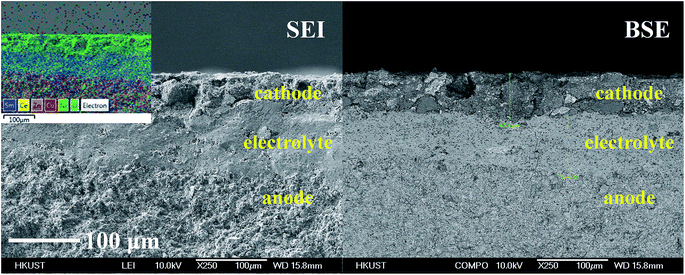 | ||
| Fig. 5 The SEM image of the fabricated button cell (left image), the back scattering electron (BSE) image (right image), and the EDX image in mapping mode (left corner). | ||
The elemental distribution in the cell was also investigated via EDX mode. The acquired images are also displayed in Fig. 5. The elemental distribution indicates that Cu and Zn were only found in the anode, and Ni was only found in the cathode. The highest intensity of Ce and Sm was found in the electrolyte.
The micro-structure analysis of the synthesized SDC and CZO indicates that the ideal micro-structure of the electrolyte and anode materials was formed during the synthesis. Sufficient surface areas of the SDC and CZO were exposed due to their fine particle size, as observed in the SEM images. The well-prepared morphology of the fabricated button cell cross-section was achieved.
3.2 Electrochemical characterization
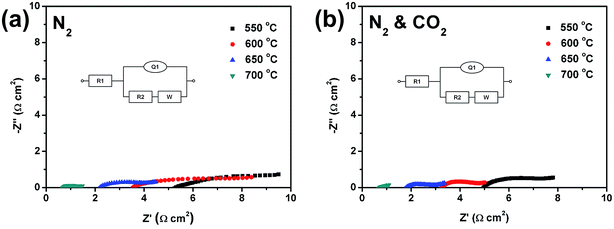
The Nyquist plots were plotted, as shown in Fig. 6a and b. It can be clearly seen that higher operating temperatures led to lower cell impedance. All the plots in Fig. 6 consist of two parts: a compressed semicircle and a shoot-up linear part, which refer to the cell internal impedance and the Warburg-type diffusion resistance, respectively. Circuit simulations were also applied to the Nyquist plots to acquire the values of each element.
The typical model of the Randles circuit, which refers to the equivalent circuit of the electrode reaction (anodic reaction in this study), was applied to the simulations.15 The model's circuit is also shown in Fig. 6, in which R1 refers to the internal resistance of the cell (mainly from the electrolyte), R2 and Q refers to the charge transfer resistance and the constant phase element (CPE), respectively. W is the Warburg element, which can evaluate the diffusion of oxygen ions (the charge carriers in the internal circuit) through the electrolyte. The values of each element obtained from the simulations are listed in Table 1. It shows clearly that the overall resistance was mainly attributed to the cell internal resistance, which was almost the same resistance as the SDC electrolyte.
| Item | R1/Ω cm2 | R2/Ω cm2 | W/Ω cm−2 | Q/Ω cm2 | θ/deg | Err | |
|---|---|---|---|---|---|---|---|
| N2 | 550 °C | 5.22 | 3.44 | 0.412 | 4.14 × 10−3 | 39.1 | 0.053 |
| 600 °C | 3.54 | 2.71 | 0.384 | 3.92 × 10−3 | 36.1 | 0.036 | |
| 650 °C | 2.27 | 2.02 | 0.300 | 2.25 × 10−3 | 34.2 | 0.060 | |
| 700 °C | 0.707 | 0.490 | 0.169 | 1.08 × 10−3 | 49.3 | 0.036 | |
| N2 & CO2 | 550 °C | 4.98 | 2.96 | 0.400 | 4.71 × 10−3 | 31.1 | 0.028 |
| 600 °C | 3.23 | 1.87 | 0.327 | 3.89 × 10−3 | 33.8 | 0.016 | |
| 650 °C | 1.88 | 1.05 | 0.289 | 2.36 × 10−3 | 41.3 | 0.022 | |
| 700 °C | 0.707 | 0.279 | 0.0705 | 9.44 × 10−4 | 81.9 | 0.062 | |
It can be observed that the contact resistance between the carbon fuel and the anode was small, due to the low R2 and W values when compared to R1, which indicates that the charge and mass transfer between the carbon fuel and the anode were in good conditions. This favored the anodic reaction which was the rate-determining step (RDS) of the carbon electro-oxidation reaction in the anode. It might prove that the CZO and SDC were mixed well when forming the composite anode, with sufficient three-phase boundaries (TPBs) among the CZO, SDC, and the carbon/molten carbonate slurry. These sufficient TPBs can provide plenty of reactive sites for the anodic electro-oxidation reactions of carbon fuels.
It can also be examined that when the operating temperature increased from 550 °C to 650 °C, the ratio of the sum resistance of R2 and W and the total resistance increased as well.15,23 This indicates that the conditions of the charge and mass transfer dominated the electro-chemical performance of the cell at higher operating temperatures. The ratio decreased when the operating temperature reached 700 °C. This might be caused by the fast carbon gasification in the Boudouard reaction (reaction (9)) that was activated at this high temperature. Since CO was sufficiently produced by the Boudouard reaction, it replaced the solid carbons as the main direct fuel for carbon electro-oxidation in the anode.24 Besides the direct contact between solid carbons and the anode (or the enhanced interface between the carbon/molten carbonate slurry and the anode), it introduced the gas phase as the new way to transport carbon fuels, which strongly enhanced the charge and mass transfer of the anodic reactions. Therefore, the internal resistance of the cell (R1) became dominant, and this caused the decrease of above mentioned ratio.
Low reactance (Q) values and relatively small phase angles (θ) were obtained in the simulation results of Nyquist plots. The values of reactance (Q) were smaller in 2 or 3 orders of magnitude than those of resistance (R1 & R2). Relatively small phase angles (θ) indicated the cell circuit performed more like a pure resistance circuit. In addition, all the simulations implemented small errors (Err) compared with the measured data, which indicated that good fitting results were achieved.
 | ||
| Fig. 7 The I–V–P plots of the single button cell fed with carbon black, under the inert environment of pure N2 and N2 & CO2 operating at (a) 650 °C and (b) 700 °C. | ||
It can be concluded that high operating temperature does promote cell performance. Since both the internal resistance of the cell and the resistance of charge and mass transfer decreased when the operating temperature increased, the total resistance of the cell also decreases, as demonstrated in Table 1. Thus, an increasing current could be obtained to achieve a better cell performance at high operating temperatures compared to that at low operating temperatures.
Through further analysis of Fig. 7a and b, the I–V–P plots indicate that when CO2 was fed to the anode, the maximum power density increased by ∼23% at 650 °C and ∼25% at 700 °C, respectively. It can be seen from Fig. 7 that at the same cell voltage, the current density increased with CO2 supplied to the anode. In addition, the phenomena became more significant at higher current density range. Thus, the higher cell outputs in power density were mainly attributed to this higher current density compared to that when pure N2 was supplied to the anode, especially for the high current density range. CO2 supply was the cause of this higher current density.
The improvement of cell performance with CO2 in the anode might be due to the following reasons. First, the supplied CO2 might combine with the oxygen anions (O2−), which are generated from the cathodic reducing reaction, to form carbonate anions (CO32−). Then, the generated carbonate anions stabilize and dissolve in the carbon/molten carbonate slurry, which in turn oxidizes the carbon fuels electro-chemically to regenerate CO2.18 In short, CO2 participates in the formation of the reaction intermediates (CO32−) as the charge porter and the catalyst. This mechanism promotes the transport of oxygen anions (O2−) to the anode zone, and extends the reactive zone of carbon electro-oxidation from the near anode region to the bulk carbon/molten carbonate slurry.25 Thus, it improves the cell performance.
Second, CO2 reacts with solid carbon fuels chemically via the Boudouard reaction to form CO, which is also the anode fuel of DCFCs.5,15,25 Since CO is in gas phase, the electro-oxidation of CO in the anode site occurs easier and faster than that of solid carbon fuels, due to the lower resistance of mass transfer, especially for the cell outputs at the high current density. In Fig. 7a and b, this could be confirmed by the higher current density with CO2 supply compared to that without CO2 supply at the same operating voltage and temperature. In this situation, the main electro-oxidation reaction might be changed from direct carbon oxidation to direct CO oxidation. The solid carbon fuels are decomposed by CO2 to sustain the CO consumption. Meanwhile, the Boudouard reaction also fastens the decomposition of large carbon grains into small fine particles, which increases the reactive sites of the carbon fuels by exposing more surfaces to the anode.
It can also be observed in Fig. 7a and b that when CO2 was supplied to the anode, the OCV values dropped slightly compared to that without CO2. This is consistent with the previous reports in literature.17,18 The reasons might be due to the different mechanisms of carbon electro-oxidation and the differences of CO2 partial pressure with and without CO2 supply to the anode. When pure N2 was supplied to the anode, solid carbon was the main reactant involved in the reaction of carbon electro-oxidation. When CO2 was supplied to the anode, CO generated via the Boudouard reaction was deemed to be the main reactant to join the carbon electro-oxidation. The theoretical OCV value of this reaction was slightly lower than that of the direct electro-oxidation of carbon.18 Furthermore, when CO2 was supplied to the anode, the partial pressure of CO2 increased as well. From the Nernst equation as shown in eqn (10), it can be concluded that the partial pressure of CO2 is negatively correlated to the theoretical OCV value.26 Therefore, the supply of CO2 to the anode causes the decrease in OCV values.
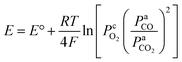 | (10) |
The cell performance test indicates that excellent cell performance was achieved using the CuO–ZnO–SDC composite as the anode, while high operation temperature and CO2 content in the anode promoted the cell performance.
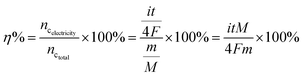 | (11) |
After the EIS measurement and the performance test, the fuel utilization was also measured under the inert environment of N2 & CO2 at 650 °C. The fuel utilization can be calculated through eqn (11). Here, i and t refer to the current and the time of the fuel cell output, respectively; F refers to the Faraday constant; m and M refer to the mass and the molecular weight of carbon, respectively. The cell was loaded under a constant current density (50 mA cm−2) and the voltage was recorded as a function of time with the chronopotentiometry technique. The result was plotted as shown in Fig. 8. It can be observed that the voltage was nearly stable from the initial value of 0.78 V to ∼0.73 V during the first 3 hours. Then, the voltage dropped to ∼0.45 V at a nearly constant rate in the following 2 hour, and finally, the voltage dropped to less than 0.3 V after 7 hours. Due to the carbon fuel depletion, a cell life of ∼8 hours was achieved in the fuel utilization test. Through Fig. 8, the calculated fuel utilization was 11.19%, which indicates that a CuO–ZnO–SDC composite performs well as the anode of DCFCs.26
4 Conclusions
In this study, a DCFC with a CuO–ZnO–SDC (Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn in a molar ratio of 1
Zn in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) composite anode was demonstrated. Nano-sized SDC electrolyte and CuO–ZnO anode materials were synthesized and characterized by diverse techniques. The synthesized materials were used to fabricate a button cell by a co-press and co-sintering method. The single button cell was tested using an anode fuel of the mixture of carbon black and ternary (Li
4) composite anode was demonstrated. Nano-sized SDC electrolyte and CuO–ZnO anode materials were synthesized and characterized by diverse techniques. The synthesized materials were used to fabricate a button cell by a co-press and co-sintering method. The single button cell was tested using an anode fuel of the mixture of carbon black and ternary (Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na
Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K in a molar ratio of 3
K in a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) eutectic carbonates (melting temperature ∼ 450 °C). The achieved maximum power density was 130 mW cm−2 at 700 °C and a cell life of ∼8 hours was achieved in the fuel utilization test at 650 °C with a mixture of N2 & CO2 (1
4) eutectic carbonates (melting temperature ∼ 450 °C). The achieved maximum power density was 130 mW cm−2 at 700 °C and a cell life of ∼8 hours was achieved in the fuel utilization test at 650 °C with a mixture of N2 & CO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volumetric ratio) as the anode gas.
1 volumetric ratio) as the anode gas.
Acknowledgements
This study was supported by the Shandong Jieda Chemical Company Ltd., Dongying, Shandong, China. The authors express their gratitude to Dr Jianbo Xu and Mr Haifeng Duan for their kind help in this work.References
- D. X. Cao, Y. Sun and G. L. Wang, J. Power Sources, 2007, 167, 250–257 CrossRef CAS.
- B. Cantero-Tubilla, C. Xu, J. W. Zondlo, K. Sabolsky and E. M. Sabolsky, J. Power Sources, 2013, 238, 227–235 CrossRef CAS.
- T. M. Gür, Chem. Rev., 2013, 113, 6179–6206 CrossRef PubMed.
- S. Giddey, S. P. S. Badwal, A. Kulkarni and C. Munnings, Prog. Energy Combust. Sci., 2012, 38, 360–399 CrossRef CAS.
- W. Hao, X. He and Y. Mi, Appl. Energy, 2014, 135, 174–181 CrossRef CAS.
- N. J. Cherepy, R. Krueger, K. J. Fiet, A. F. Jankowski and J. F. Cooper, J. Electrochem. Soc., 2005, 152, A80–A87 CrossRef CAS.
- W. A. G. McPhee, M. Boucher, J. Stuart, R. S. Parnas, M. Koslowske, T. Tao and B. A. Wilhite, Energy Fuels, 2009, 23, 5036–5041 CrossRef CAS.
- A. Jayakumar, R. Küngas, S. Roy, A. Javadekar, D. J. Buttrey, J. M. Vohs and R. J. Gorte, Energy Environ. Sci., 2011, 4, 4133 CAS.
- A. K. S. Giddey, C. Munnings and S. P. S. Badwal, J. Power Sources, 2015, 284, 122–129 CrossRef.
- W. Hao and Y. Mi, Energy, 2016, 107, 122–130 CrossRef CAS.
- Z. Cheng and M. Liu, Solid State Ionics, 2007, 178, 925–935 CrossRef CAS.
- T. Tao, L. Bateman, J. Bentley and M. Slaney, ECS Trans., 2007, 5, 463–472 CAS.
- A. C. Rady, S. Giddey, S. P. S. Badwal, B. P. Ladewig and S. Bhattacharya, Energy Fuels, 2012, 26, 1471–1488 CrossRef CAS.
- Y. H. Huang, R. I. Dass, Z. L. Xing and J. B. Goodenough, Science, 2006, 312, 254–257 CrossRef CAS PubMed.
- A. C. Rady, S. Giddey, A. Kulkarni, S. P. S. Badwal, S. Bhattacharya and B. P. Ladewig, Appl. Energy, 2014, 120, 56–64 CrossRef CAS.
- A. Kulkarni, S. Giddey, S. P. S. Badwal and G. Paul, Electrochim. Acta, 2014, 121, 34–43 CrossRef CAS.
- L. Deleebeeck, D. Ippolito and K. K. Hansen, Electrochim. Acta, 2015, 152, 222–239 CrossRef CAS.
- C. Jiang, J. Ma, A. D. Bonaccorso and J. T. S. Irvine, Energy Environ. Sci., 2012, 5, 6973 CAS.
- R. Raza, PhD, Royal Institute of Technology, KTH, Sweden, 2011.
- R. Raza, X. Wang, Y. Ma and B. Zhu, J. Power Sources, 2010, 195, 8067–8070 CrossRef CAS.
- L. Jia, Y. Tian, Q. Liu, C. Xia, J. Yu, Z. Wang, Y. Zhao and Y. Li, J. Power Sources, 2010, 195, 5581–5586 CrossRef CAS.
- M. Mogensen, N. M. Sammes and G. A. Tompsett, Solid State Ionics, 2000, 129, 63–94 CrossRef CAS.
- S. L. Jain, J. Barry Lakeman, K. D. Pointon, R. Marshall and J. T. S. Irvine, Energy Environ. Sci., 2009, 2, 687–693 CAS.
- C. Li, Y. Shi and N. Cai, J. Power Sources, 2010, 195, 4660–4666 CrossRef CAS.
- M. Powell, K. Meinhardt, V. Sprenkle, L. Chick and G. McVay, J. Power Sources, 2012, 205, 377–384 CrossRef CAS.
- Y. Jiao, J. Zhao, W. An, L. Zhang, Y. Sha, G. Yang, Z. Shao, Z. Zhu and S.-D. Li, J. Power Sources, 2015, 288, 106–114 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |