Heterogenization of heteropoly compounds: a review of their structure and synthesis
E. Rafiee
*ab and
S. Eavani
*a
aDepartment of Inorganic Chemistry, Faculty of Chemistry, Razi University, Kermanshah, 67149, Iran. E-mail: ezzat_rafiee@yahoo.com; e.rafiei@razi.ac.ir; sara_eavani@yahoo.com; Fax: +98 83 34274559; Tel: +98 83 34274559
bInstitute of Nano Science and Nano Technology, Razi University, Kermanshah, 67149, Iran
First published on 26th April 2016
Abstract
The application of catalysis to reduced toxicity systems and benign and renewable energy systems is a central focus area for green chemistry research. It is possible to prepare heterogeneous analogues of the most commonly used homogeneous soluble catalysts by immobilizing them on various insoluble supports. The use of heterogeneous catalysts in chemical processes would simplify catalyst removal and minimize the amount of waste. Therefore, to maintain economic viability, a suitable heterogeneous system should not only minimize the production of waste, but should also exhibit activities and selectivities comparable or superior to the existing homogeneous route. Accordingly, catalysis by heteropoly (and related) compounds (HPCs) is a field of increasing importance, particularly concerning nanocatalysts. Furthermore, the heterogenization of bulk HPCs is an interesting area of research from an industrial point of view. As a rapidly growing and increasing field, HPC catalysis exhibits three main merits: (1) HPCs not only possess a strong acidic property, but also an oxidative property, which can support fast reversible multi-electron redox transformations under mild conditions. (2) HPCs exhibit fairly high thermal stability in supported or salt forms. (3) Their catalytic properties can be tuned in a wide range by changing their chemical compositions. A number of important heterogenization methods will be discussed in this review. These methods can be classified into two major categories: exchange of the HPC protons with cations (precipitation and hybridization) and immobilization of the HPCs on a suitable solid support (encapsulation, grafting, tethering and dispersion). Although these compounds have been known well for over a century, only in the last few years has scientific interest in these materials begun to increase dramatically. Therefore, in this review, we aim to describe the different methods of heterogenization of these compounds developed and reported over the past 15 years.
1. General aspect
Heteropoly compounds (HPCs) or heteropoly acids (HPAs) constitute an extremely large and diverse class of polyoxometalates (POMs) containing one or more p-, d- or f-block “heteroatoms”, d0 and/or d1 transition metal cations (addenda atoms) and oxide anions. The building blocks of HPCs comprise a central heteroanion (XO4, X = P5+, As5+, Si4+, Ge4+, B3+, etc.) surrounded by transition metal oxides with a general formula {MOx}n, where M = Mo, W, V and sometimes Nb and x = 4–7.1 Since 1933, hundreds of HPCs with different structures have been synthesized, characterized and used in many diverse areas. Among these, Keggin (Xn+M12O40(8−n)−), Anderson–Evans (Xn+M6O24(12−n)−) and Wells–Dawson (X2n+M18O62(16−2n)−) structures have attracted considerable interest as catalysts.2–8The catalytic properties of the above-mentioned HPCs can be controlled by suitable selection of the heteropolyanion and its constituents, including heteroatoms, addenda atoms and counter cations. Lacunary species and transition metal-substituted HPCs are other structural derivatives that are exploited in catalysis. Lacunary species (most commonly with one, two or three vacancies) are formed by the removal of one or more addenda atoms from Keggin and Wells–Dawson anions. These anions have been used for preparation of hybrid POM compounds. Transition metal-substituted polyanions are designed by the replacement of one or more addenda atoms and the terminal oxo-group by a transition metal atom cation. The transition metal cations possess five bridging oxygen atoms, while the sixth coordination site has been shown to be occupied by a water molecule and is considered to serve as an entry to the inner-sphere electron transfer pathways, which are usually inaccessible in the unsubstituted anions. Though they are completely inorganic in nature, these compounds have been shown to exhibit catalytic chemistry similar to metalloporphyrins.
The extreme variability of the compositions, specific molecular architectures, strong Brönsted acidities, suitable redox potentials and high thermal stabilities along with low volatilities and low corrosive properties of HPCs make them suitable for green technology applications. Based on this, the synthesis, characterization and application of new HPC-based catalysts constitute exceptionally active and fast-developing fields. Although homogeneous HPC catalysts are remarkably efficient, they share a common drawback: separation and reuse of the catalyst is extremely difficult. For this reason, future practical applications of HPCs will also require methods for “catalyst engineering” to aid in catalyst recovery and recycling. In the last two decades, many research groups have focused on the replacement of homogeneous HPC catalysts with heterogeneous analogues, which can be easily recovered from the reaction mixture, thereby minimizing the amount of waste formed. The present review article highlights the increasing interest in HPCs in heterogeneous catalysis and provides a perspective for researchers in the development of new strategies for the recycling of HPC-based catalysts.
2. Heterogenization of HPC catalysts: why and how?
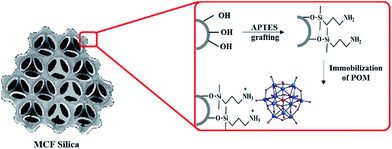
The development of new strategies for the recycling of catalysts is a task of great economic and environmental importance in the chemical and pharmaceutical industries. Although HPCs are used as heterogeneous catalysts in non-polar solvents, their high solubility in many polar solvents renders them into their homogeneous phase. In this case, heterogenization is necessary to prevent this. Increasing the amount of accessible surface active sites is another goal that could be considered in the preparation of heterogeneous HPC catalysts. Although HPAs are efficient homogeneous catalysts, the amount of active sites on their surface is small because of their low surface area (5–10 m2 g−1). In this case, non-polar reactant molecules cannot diffuse in to the pseudo-liquid phase of the HPAs and are adsorbed only on their external surface. This mechanism is called “surface-type catalysis”, where the reactions take place on the two-dimensional (2D) surface (outer surface and pore wall) of the solid catalysts, and the reaction rate is, in principle, proportional to the surface area. Therefore, for many heterogeneous catalytic applications, the immobilization of HPAs onto a high surface area carrier is desirable.It is possible to prepare heterogeneous analogues of HPCs by the dispersion of HPAs on different insoluble supports. These catalysts can be recycled simply by filtration or centrifugation. However, in some cases, weak interaction between the HPAs and the supports lead to the leaching of HPA from the support surface in polar reaction media. In order to resolve this problem, other strategies, including encapsulation, grafting and tethering, have been developed (Fig. 1). These strategies will be discussed in the following section. Using these methods, the leaching of HPAs is negligible, but, compared to the homogeneous cases, lower activities or selectivities are commonly detected due to steric and diffusion factors. However, when the size of the support material is decreased to the nanometre scale, the surface area of the support increases dramatically, and, as a consequence, nanoparticles have a higher catalyst loading capacity and a higher dispersion than many conventional supports, leading to improved catalytic activity. The strategy of magnetic separation is typically more effective than filtration or centrifugation for catalyst recovery as it prevents loss of the catalyst. Therefore, currently, much attention is focused on the design and synthesis of nano-magnetically recyclable HPC-based catalysts.
Another alternative approach for the heterogenization of HPCs is to prepare insoluble HPA salts by partially or completely exchanging protons of the parent HPAs with suitable cations (Fig. 1). Traditionally, large alkali metals and/or transition metals have been used for this purpose. Except for a few cases, such as Cs2.5H0.5PW12O40, these salts have a low surface area and generally produce a colloidal dispersion, and, as a result, catalyst separation is difficult. In the last decade, many improvements have been developed for the synthesis of organic–inorganic HPA salts through the different combinations of various organic cations and heteropoly anions. Consequently, the development and application of these new hybrids has emerged as one of the most potentially significant fields of investigation in catalysis. Some of these materials have been used as “reaction-induced self-separation” or “thermoregulated phase-separable”, depending on the change in the polarity or temperature of the reaction mixture, respectively. In such cases, the reaction medium switches from a homogeneous system to a heterogeneous one at the end of the reaction. Therefore, the use of these catalysts combines the advantages of a one-phase homogeneous catalysis with a facile method for catalyst separation.
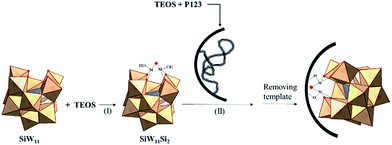
The heterogenization of HPC-based catalysts has been an important trend in catalysis research in recent years, and several ways have been reported so far. A number of important instances of such methods will be discussed in the following sections (Fig. 2). These methods can be classified into two major groups: exchange of the HPA protons with cations (precipitation and hybridization) and immobilization of the HPA on a suitable solid support (encapsulation, grafting, tethering and dispersion). In addition to recyclability, as discussed in the previous section, thermal stability and the amenability to continuous processing, and in some cases multifunctionality, are other factors that are considered in the design of heterogeneous HPC-based catalysts.
2.1. HPAs precipitation with inorganic metal cations
Water-insoluble HPA salts with certain inorganic cations exhibit dramatic changes in their surface area and pore size in comparison to the parent HPAs. These solids are prepared simply by precipitation from aqueous HPA solutions. If these solids are prepared by the partial substitution of protons, residual quantities of protons will still remain, and it is these that are responsible for the catalytic activity of these salts.9 If all the protons of the parent HPAs were substituted by the inorganic cations, the obtained “neutral” polyanion salts could also gain proton sites upon interaction with the reaction media. Two mechanisms for proton generation in neutral salts can be distinguished: acidic dissociation of coordinated water and reduction of the metal cations.10 Also, metal salts of HPA potentially show Lewis acidity, originating from the metal cations as electron pair accepters.Recently, Kamiya et al. synthesized bimodal CsxH4−xSiW12O40 with micropores and interconnected mesopores by treating microporous CsxH4−xSiW12O40 with x = 1.0–2.5 in refluxing ethanol.23 The presence of three crystallites (including H4SiW12O40, Cs2.2H1.8SiW12O40 and Cs3.7H0.3SiW12O40) in the appropriate ratio was crucial for the formation of mesopores with regular shapes in the bimodal caesium salt. In addition, the choice of solvent used for the treatment is another factor for controlling the shape and volume of the mesopores.23
The Keggin-type HPAs, i.e. H3+nPMo12−nVnO40 (n = 0–2), and their caesium salts are well-known catalysts for the partial oxidation of organic compounds by oxygen.24,25 Kozhevnikov et al. reported the use of these catalysts in the vapour-phase hydrogenation of propanoic acid.26 They also showed that the catalyst acidity has a crucial effect on the reaction selectivity. Tuning the catalyst acidity by caesium substitution in HPAs allows for the propanal selectivity to be maximized. There is compelling evidence that this reaction occurs via a redox Mars–van Krevelen mechanism, with catalyst acidity (controlled by ion exchange, e.g. with Cs+ cations) playing an important role.26 Colloidal Cs3PW12O40 was used as a photocatalyst in the photo-oxidations of a number of organic molecules.27–29
The physico-chemical and catalytic properties of caesium partly-substituted salts of Wells–Dawson HPAs, i.e. CsxH6−xP2W18O62, have also been reported;30,31 for instance, Guo et al. synthesized and characterized a series of insoluble caesium partly-substituted Wells–Dawson-type HPAs, i.e. CsxH6−xP2W18O62 with x = 1.5–6.0.31 These salts were applied to produce diphenolic acid from levulinic acid. The catalytic behaviour of Cs1.5H4.5P2W18O62 was compared with that of the Keggin-type Cs2.5H0.5PW12O40. It was found that both the activity and selectivity of Cs1.5H4.5P2W18O62 exceed those of Cs2.5H0.5PW12O40, owing to their different reaction types. For the Cs1.5H4.5P2W18O62 samples, a pseudo-liquid behaviour is considered as the governing factor for its high catalytic activity and selectivity. Also, the catalyst activity did not change significantly after three repetitive runs.31
As an interesting example, K5CoW12O40, with E0 = 1.07 V (against a normal hydrogen electrode), was used as an oxidant, both for organic and inorganic substances. It is an apparently perfect outer-sphere oxidant because the central Co(III) atom is protected by a sheath of chemically inert oxygen atoms that protect the central ion from undesired inner-sphere substitution reactions. This heterogeneous catalyst has shown excellent reactivity in different organic reactions.32–41 The possible mechanism for the catalytic addition of cyclic and acyclic diketones to alcohols in the presence of K5CoW12O40 was reported by Rafiee et al.41 The alcohol was protonated to generate a stable carbocation after dehydration, which could quickly combine with the employed 1,3-dicarbonyl compound to produce, after the release of H+, the final alkylated product. Also, due to the resonance of oxygen with the adjacent π-bonding electrons, the cyclic 1,3-dicarbonyl compound as a nucleophile combined with the stable carbocation to produce β-keto enol ethers (Scheme 1). Also, the catalyst could be used four times with only a little change in its catalytic activity.
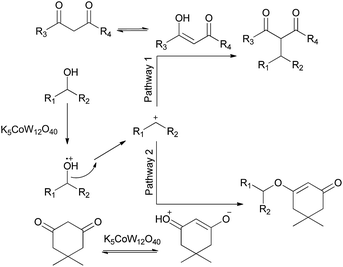 | ||
| Scheme 1 Proposed mechanism for the catalytic addition of different diketones to alcohol.41 | ||
The neutral silver salt Ag3PW12O40 is characterized by its outstanding acidity, which is about one order of magnitude higher than that for Cs3PW12O40.46 1H MAS NMR measurements of Ag3PW12O40 salt have shown protons at 9.3 ppm, resulting from the dissociation of the structural water. The same chemical shift is observed in H3PW12O40 after preheating at 473 K.47 It was observed that the counter cations in the Ag3PW12O40 salt possess the ability to dissociate molecular hydrogen, leading to the generation of active protons.48 The reduction of the silver salt with hydrogen is performed according to the equation:
| Ag+ + ½H2 → Ag0 + H+ |
Therefore, the catalytic activity of Ag3PW12O40 is greatly enhanced by the presence of hydrogen in the gas phase. The activity of reduced Ag3PW12O40 in the presence of hydrogen is much higher than that of H3PW12O40, in which the catalytic activity is not influenced by hydrogen. Besides, the dynamic properties of the protons in the Ag3PW12O40 salt play an important role in acid-catalyzed reactions.49–51
Mucha et al. reported detailed structural studies on Ag3PW12O40 using XRD measurements and the Rietveld refinement, as well as XPS, EDX and FT-IR techniques.51 They showed that Ag3PW12O40 salt forms a cubic structure similar to the structures of Cs3PW12O40. However, the basic difference between them lies in hydration state of the Ag+ ion and in its asymmetric position in the octahedral space between the Keggin anions. This causes the neutral silver salt to form two different structures: the first one exists up to 473 K while the other one appears above 573 K. At 473 K, the [Ag(H2O)]+ complex is centred in the octahedral space between the Keggin anions. Each silver cation is bonded to two terminal oxygens of different Keggin anions, while the water molecule forms weak hydrogen bonds with the two other Keggin anions. Surprisingly, in the structure of the Ag3PW12O40 salt existing at 573 K, the dehydrated silver cation Ag+ is not centred in the octahedral space between Keggin anions, and instead the silver cation occupies statistically one of four 24f Wyckoff positions. This results in a large possibility of dimer Ag–Ag formation, with bond lengths even as short as 2.62 Å.49 Hill et al. synthesized Ag5PMo10V2O40 salts for the first time and reported its better stability and catalytic activity than Na5PMo10V2O40 (soluble salt).52
Many research groups have focused on the bifunctional catalysts based on noble metal salts of Keggin-type HPAs and investigated their catalytic performances in various organic transformation. The application of palladium salts of HPAs (Pd–HPA) is very attractive, as in this case the palladium centre and the redox component are joined in one complex. This ensures direct contact between the palladium site and HPA, thus facilitating the transfer of electrons to the HPA. The application of Pd–HPA also simplifies the preparation of the catalysts, since both the palladium and redox component can now be deposited in one impregnation step. Stobbe-Kreemers et al. described the catalytic cycle of Pd–HPA in 1-butene oxidation (Pd0H2–HPA), representing the reduced palladium salts:53
| PdII–HPA + C4H8 + H2O → Pd0H2–HPA + C4H8O |
| Pd0H2–HPA + ½O2 → PdII–HPA + H2O |
The oxidation of arenes by Pd(II) has attracted considerable interest. These reactions appear to involve the electrophilic substitution of arene by Pd(II) to form an σ-arylpalladium(II) intermediate.54,55 The oxidation can be made catalytic by reoxidizing Pd(0) back to Pd(II). Oxygen can deoxidize Pd(0) directly, though it needs elevated temperatures and pressures. The catalytic oxidation occurs easier when mediated by HPCs as redox co-catalysts,56,57 similarly to the Wacker-type oxidation of alkenes.58
Pd-containing compounds are traditional catalysts for C–C and C–N coupling reactions. The combinations of Pd compounds and HPAs have received much attention. HPAs act as co-catalysts for the regeneration of Pd(II), and the reduced HPAs are reoxidized by O2 in the next step.59–64
The copper, zinc, cobalt, iron and manganese salts of Keggin-type HPAs have also been used as catalysts for various organic reactions.65–78 Filek et al. reported the use of reduced copper salts of Wells–Dawson-type HPAs, i.e. Cu3P2W18O62, as a bifunctional catalyst for ethanol conversion.79 XPS measurements indicated the presence of both Cu(0) and Cu(I) species. In oxygen-free helium, the typical reaction products are formed on Brönsted acid centres (including ethylene, diethyl ether and water) supplied by free HPA. On the other hand, in the presence of air, acetaldehyde was predominantly obtained in the catalytic reaction. Such a reaction occurs on redox centres and is ascribed to the presence of catalytic redox centres formed by the reduced copper.
Shimizu et al. used the metal salts of H3PW12O40 (including Y3+, Al3+, Zr4+, Ti4+, Hf4+ and Sn4+) as catalysts in the alkylation of toluene with iso-propanol.80 It was found that the activity increased with the electronegativity of the cation, and that Sn4+ and Hf4+ salts showed higher catalytic activities than the others. This suggested that the catalytic efficiency increases with the Lewis acid strength, and the rate correlated well with the number of Lewis acids, indicating that the Lewis acid sites originating from the Sn4+ cation were responsible for the alkylation reaction.
The use of multivalent metal (Ti4+, Fe3+, Sn4+, Bi3+, Ru3+) salts of H3PW12O40 in the Friedel–Crafts acylation of aromatics with carboxylic acids was also reported by Shimizu's group.81 The rate of acylation depended strongly on the Lewis acidity originating from the exchanged metal cations.
Jie et al. reported the use of a Ce3+ salt of a Dawson-type tungstophosphoric acid (Ce2P2W18O62·16H2O) in the synthesis of n-butyl acetate with acetic acid and n-butanol.86 The results of pyridine infrared spectroscopy indicated that the catalyst possessed both Brönsted and Lewis acid sites. The catalysts could be recycled and still exhibited high catalytic activity, with a 90.4% conversion after five cycles of reaction.
Wang et al. used Agx(NH4)5−xPMo10V2O40 as a catalyst in starch oxidation.89 Among all the compounds, Ag3.5(NH4)1.5PMo10V2O40 exhibited the highest activity, with a high oxidative degree of 0.62 mol per 100 g due to its surface area and due to the synergistic effect of the Ag and V cations. It also worked as a heterogeneous catalyst, which allowed it to be easily recycled and reused.
Pd0.15Cs2.5H0.2PW12O40 was applied to the direct synthesis of hydrogen peroxide from hydrogen and oxygen.90 It showed high catalytic performance even in the absence of the H2SO4 additive, indicating that it could act as an efficient catalyst and serve as an alternate acid source in the reaction.
Liu-Cai et al. investigated the catalytic activity and selectivity of the CsxH1−xVO[PMo12O40] and CsyH0.5−yCu0.25VO[PMo12O40] salts in the oxidation of isobutane as a function of the Cs+ content.91
Millet's group designed a new catalyst by the partial substitution of protons in Cs2HPMo12O40 by tellurium and vanadium cations.92,93 The Cs2TexVyPMo12O40 catalysts were used in the partial oxidation of isobutane into methacrylic acid. Tellurium was selected for its hydrogen abstracting efficiency and showed a strong effect on the selectivity to methacrylic acid. The concomitant substitution of protons by the vanadyl cations allowed increasing the activity of the catalysts without considerable decreasing the selectivity to methacrylic acid.
2.2. Hybridization
Recently, many improvements have been achieved by the synthesis of new hybrid materials through the different combinations of various complexes and organic or organometallic cations with HPA anions. Consequently, these new hybrids have emerged as one of the most potentially significant fields of investigation in catalysis today. The controlled assembly of POM-based building blocks represents a crucial challenge to engineer the POM building blocks in such a way that they can assemble into novel architectures with desired functionality. An important extension to this building block concept is realized by the use of POMs to form organic–inorganic hybrid compounds, which comprise covalently connected cluster and organo fragments, thereby allowing the intermit combination of the properties of the metal–oxo and organic building blocks; this has been used to prepare polymers, dendrimers and macroporous materials. It is apparent now that organic components can dramatically influence the microstructures of inorganic oxides, thus providing a route for the design of novel materials, and this is widely shown in the natural world. Such organic–inorganic hybrid materials not only combine the advantages of organic molecules, such as structural fine tuning, but also the close interaction and synergistic effects of the organic group and inorganic cluster. According to the abovementioned discussion, it could be better to divide these materials into three main categories. Of course there are other different forms of hybrid materials based on POM compounds but these three categories are the most synthetized ones.94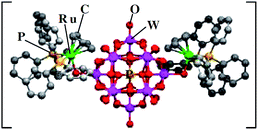 | ||
| Fig. 3 Ru-tethered POM Keggin anion. Reproduced from ref. 97 with permission from Elsevier. | ||
A novel organometallic-POM hybrid was prepared by the anion-exchange of a V Schiff base functionalized ionic liquid (IL) with a V-containing Keggin-type POM (Fig. 4). The hybrid solid, containing two types of catalytic active V components, demonstrated a remarkable capability for the heterogeneous hydroxylation of benzene, with an excellent phenol yield of 19.6% and 100% selectivity. The synergistic effect between the metal Schiff base complex and POM plays an important role in promotion of the catalytic activity.98
As an example a new inorganic–organic complex [Cu(phen)2]2PVW11O40 was formed by utilizing POMs. The polyanion unit, PVW11O404−, in which every heavy-atom position is occupied by 11/12 W and 1/12 V, acts as an inorganic ligand coordinating to two Cu(II) atoms through one terminal and one bridging oxygen atom, respectively. Furthermore, the molecules stack with each other to form a 3D structure due to the C–H⋯O hydrogen bonds and π–π stacking interactions (Fig. 5).99
 | ||
| Fig. 5 A new inorganic–organic complex [Cu(phen)2]2PVW11O40. Reproduced from ref. 99 with permission from Elsevier. | ||
Three pure inorganic eight-connected self-catenated networks based on the Silverton-type POM [CeMo12O42]8− with lanthanide, transition metal and alkali metal cations as linkers: [Li(H2O)4]2Co(H2O)4Ce(H2O)3[CeMo12O42]·3H2O, H0.5[Li(H2O)4]2.5[Ni(H2O)4]0.5Ce(H2O)3[CeMo12O42]·3H2O and H[Li(H2O)4]3Ce(H2O)3[CeMo12O42]·3H2O were successfully synthesized by H. Tan et al. Single-crystal X-ray diffraction analyses revealed that all three compounds were isostructural (Fig. 6).100
 | ||
| Fig. 6 Inorganic eight-connected self-catenated networks based on the Silverton-type POM. Reproduced from ref. 100 with permission from Elsevier. | ||
Another hybrid compound was hydrothermally synthesized by using a cobalt complex as a new bicapped Keggin-type HPA derivative as [Co(bpy)3]2[PMoVI8VV3VIVO40(VIVO)2] [{Co(bpy)2(H2O)}2{PMoV8VV3VIVO40(VIVO)2}]·6H2O. The most interesting feature of this hybrid compound is that a polyoxoanion and a neutral component coexist in the crystal structure. Hydrogen bonding contacts are also formed between the different polyanions through the lattice water molecules (Fig. 7).101
The synthesis of tailored multimetal catalysts is becoming increasingly important for their high efficiency and multifunctionality in various applications. A totally inorganic oxygen-evolving catalyst was synthesized by the template-directed metalation of [γ-SiW10O36]8− using tetraruthenium(IV)-oxo-core (Fig. 8).102
 | ||
| Fig. 8 Metalation of SiW10 by complementary Lego assembly.102 Reprinted with permission from J. Am. Chem. Soc., 2008, 130, 5006. Copyright © 2008 American Chemical Society. | ||
A monovacant Keggin-type POM-supported trirhenium carbonyl derivate, [(CH3)4N]5H23[(PW11O39){Re(CO)3}3(μ3-O)(μ2-OH)]4·24H2O, was prepared as an efficient catalyst for cyclic carbonate and epoxide synthesis.103
Six novel organic–inorganic hybrid derivatives with two types of organic ligands were hydrothermally synthesized and structurally characterized. Furthermore, the photocatalysis properties of rhodamine-B under 500 W Hg lamp irradiation in the presence of these catalysts were examined (Fig. 9).104
 | ||
| Fig. 9 Polyhedral/ball-and-stick representation of (a) structural unit and (b) 1D chain built by alternate [Ce(R-PW11O39)2]11− units, [Cu3(en)2]2+ and [Cu2(en)(2,20-bipy)]2+ bridges.104 Reprinted with permission from Cryst. Growth Des., 2011, 11, 3769. Copyright © 2011 American Chemical Society. | ||
Magnetite–POM hybrid nanomaterials, Fe3O4/SiO2/salen/Mn/IL/PW12, were prepared by grafting H3PW12O40 onto IL-functionalized Fe3O4 magnetite nanoparticles (Fig. 10).105
 | ||
| Fig. 10 Magnetite–POM hybrid nanomaterials. Reproduced from ref. 105 with permission from Springer. | ||
Some of these organic ligands use phosphorous as the donating atoms and some use nitrogen. As an example, the pairing of a Keggin or Lindqvist POM anion with an appropriate tetraalkylphosphonium cation yielded the first members of a new family of ILs. Detailed characterization of one of them, an ambient-temperature “liquid POM” comprising the Lindqvist salt of the trihexyl(tetradecyl) phosphonium cation, by voltammetry, viscometry, conductimetry and thermal analysis showed that it exhibited conductivity and viscosity comparable to those of the previously described inorganic–organic POM–IL hybrid but with substantially improved thermal stability.106,107
There are more reported publications with nitrogen as the coordinating atom. There are also some publications using imidazolium hybrid ILs as photocatalysts. Certain interesting structural and electronic features of the BMIm-based ILs, such as: (i) the ability to provide the reaction system with a radical or cation propagating source (the imidazolium moiety); (ii) as in ILs, the ability to form an extended hydrophobic channel-like compartments, which can stabilize the emerging oligomers,108,109 led to the choice of BMIm+/DMIm+ as the second component.108–110 In short, the design strategy of such catalysts exploits: (i) the photoactivity of POM coupled with the presence of imidazolium to catalyze radical/cationic polymerization initiation; (ii) the advantages of the imidazolium moiety; (iii) the stabilization of the emerging oligomeric chains in the extended hydrophobic compartments by the dangling butyl chains, thus rendering the catalyst functional and (iv) the redox recoverability of POM under the stipulated reaction conditions.111
Such complexes combine the flexibility and reactivity of HPAs with the tunable solvency and task-specific property of ILs and, thus, have been used as, depending on the selected reaction media, liquid–liquid biphasic catalysts, reaction-induced self-separation catalysts or heterogeneous catalysts in various organic transformations. More importantly, imidazolium cations have, in some cases, shown the ability of activating HPAs, leading to elevated catalytic efficiency.112–133
A series of Anderson-type Q4NiMo6−xWxO24H6 (x = 0, 2, 4, 6) catalysts were synthesized and employed in ILs extraction coupled with catalytic oxidation desulfurization systems for the removal of sulfur compounds in a model diesel fuel and in an actual commercial diesel (Fig. 11).134,135
Some of the POM-based organic–inorganic hybrid materials include amphiphilic catalysts that have been synthesized by combining quaternary ammonium cations and POM anions.136–140 These compounds have the ability to form emulsion droplets, which perform as homogeneous catalysts in the interface of two immiscible liquids to help achieve high activities during the oxidative process. However, the de-emulsification for catalyst recovery is often challenging in a large-scale unit. Catalyst recovery in an active form suitable for recycling is generally not feasible and the products may be contaminated with the catalyst residues. This situation often leads to the need for expensive purification procedures, which is at odds with the development of more sustainable processes. Recently, to solve this problem, a novel thermoregulated phase-separable catalyst was introduced for the selective oxidation of organic sulfur compounds in aqueous media.141–144 These temperature-dependent systems not only showed excellent catalytic performance, but also provided a convenient way to reuse and avoid the leaching of catalysts from the solvents.
A new kind of POM–IL, [(CH3)3NCH2CH2OH]5PV2Mo10O40, was synthesized by a precipitation/ion exchange method with choline chloride and H5PMo10V2O40 as the precursors. The produced salt turned out to be an original IL catalyst and exhibited a novel switchable property based on temperature variation. Also, in this case, on decreasing the temperature, the catalyst precipitates and becomes in a heterogeneous form, allowing it to be separated automatically from the reaction mixture. The combination of POMs with choline chloride was also proven to be effective in catalyzing the oxidation of starch, which showed a higher or nearly identical performance with traditional catalysts, such as FeSO4.142
Some other thermoregulated IL–POMs were produced by Rafiee et al. and the activity of these catalysts were investigated in biodiesel production and in extractive oxidative desulfurizations and other reactions.141,143,145
Among these systems, the soluble polymer-based thermoregulated system has been of great interest in recent years. Based on the same concept, J. Chen et al. utilized a polymer-based thermoregulated IL as an efficient and convenient catalyst for the epoxidation of olefins in the presence of H2O2 in ethyl acetate, and attempted to solve the problem associated with the separation and recycling of the catalysts (Fig. 12).146
The structural bonding of some hybrid ILs was investigated by Zonoz and co-workers, who proved that there are two types of hydrogen bonding in compound 1: (i) the hydrogen bonding existing between the Keggin clusters and imidazole ligands; (ii) the hydrogen bonding present between the Keggin structure, the non-coordinated water molecule and the imidazole ligand. And also, there were shown to be π interactions between the imidazole ligands.147
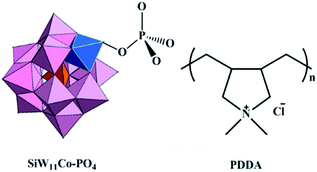
A novel nano-sized biological active multilayer film composed of a POM anion α-[SiW11O39Co(H2PO4)]7− (SiW11Co–PO4) and poly(diallyl dimethylammonium chloride) (PDDA) was fabricated by a layer-by-layer self-assembly (Fig. 13).148
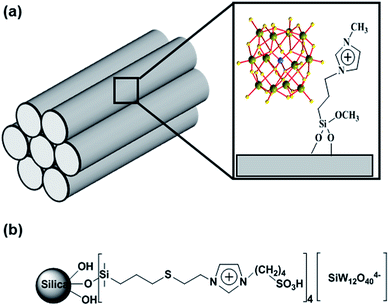
Some other kinds of polymer-based IL–POM hybrids are shown in Fig. 14.149–153
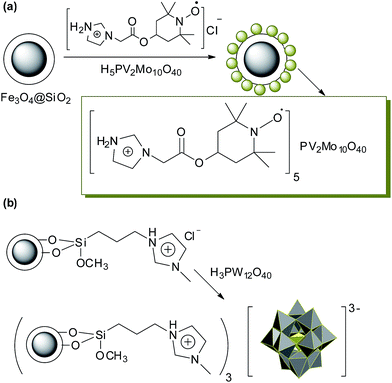
Mixed organic and metal cation salts of IL–POMs sometimes have excellent catalytic activity.154 More recently, in order to combine the merits of HPA–onium cation catalysts with supported HPA catalysts, several reports have been published describing the use of immobilized HPA–onium cation complexes as intrinsically heterogeneous catalysts. In comparison with HPA–onium cation complexes, the resultant catalysts show additional advantages, such as a decreased consumption of HPA–onium cation complexes, a facilitation of catalyst separation from the reaction system and a lower contamination of product, just like in the cases of IL heterogenization (Fig. 15a).155 Another hybrid was reported by B. Zhen et al. (Fig. 15b).156
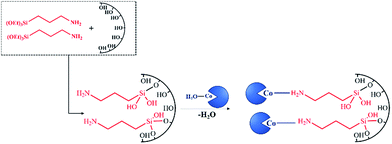
Magnetic-supported HPA–ILs were also synthesized with different methods. Two of the synthesis procedures are presented in Fig. 16.157,158
Three novel multi-SO3H–functionalized heteropolyanion-based ionic hybrids were synthesized and characterized, e.g. as heterogeneous catalysts for the Baeyer–Villiger oxidation.159 There are some other research papers in this subject area (Fig. 17).160,161
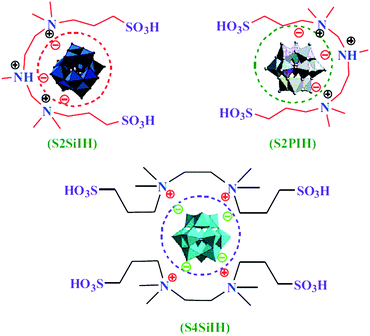 | ||
| Fig. 17 Multi-SO3H functionalized heteropolyanion-based ionic hybrids. Reproduced from ref. 159 with permission from Elsevier. | ||
2.3. Dispersion
Dispersion is the immobilization of HPA on supports that do not contain specific anchoring sites for dispersed HPA particles. Virtually any solid that has a surface area of at least 10 m2 g−1 can be used as a support. Support materials, such as silica, alumina, titania, activated carbon, magnesia, etc., have been applied with varying levels of success, with new supporting materials and methods continually being actively pursued. Acidic or neutral substances, such as silica and active carbon, are suitable as supports. Basic solids, such as alumina, titania and magnesia, tend to decompose HPA. Some recent publications reporting the application of these supports for HPAs are listed in the following sections.(i) It is widely available, is neutral or mildly acidic and possesses tuneable specific surface areas and porosity.
(ii) According to temperature programmed desorption data, the acid strength of supported H3PW12O40 decreases in the series of carriers: SiO2 > α-Al2O3 > activated carbon.
(iii) The thermal stability of HPA on SiO2 seems to be comparable to or even slightly higher than that of the parent HPA.
(iv) The thermally decomposed Keggin structure on the silica surface may be reconstructed on exposure to water vapour.
Silica-supported HPA catalysts were prepared by impregnating Aerosil 300 silica (SBET, 300 m2 g−1) with an aqueous solution of H3PW12O40 by J. Kaur and I. V. Kozhevnikova, and their catalytic activity were investigated in Friedel–Crafts acylation and in Fries rearrangement of aryl esters, respectively.162,163 During the reaction, even in non-polar solvents, such as dodecane, H3PW12O40 leached from the silica support. Significant coking was observed for the highly porous H3PW12O40/SiO2 (6–13 wt% carbon). However, the catalyst could be separated by filtration and, after a simple work-up, such as washing with dichloroethane, reused, albeit with reduced activity. The authors tried to control the reusability of the catalyst by Pd doping. They found that 1.5% Pd doping of H3PW12O40/SiO2 had little effect on the coke burning: only about a 50 °C temperature shift was observed in the TGA/TPO plot. There may be several reasons for this, such as how the coke was laid down on the highly porous H3PW12O40/SiO2. In the case of H3PW12O40/SiO2, the massive coke localized in the pores would be more difficult to burn out. In addition, Pd loading on H3PW12O40/SiO2 (1.5%) may be insufficient. It has been shown that Pd doping only becomes effective for coke burning at loadings of 2–2.5%. Sometimes with insufficient reusability, it could be better to change the washing solvent or to do the catalytic reaction in a solvent-free system.164
According to the already-mentioned advantages of silica as a support, there are a lot of research on different HPAs supported on silica by impregnation methods and that are used in various organic synthesis and industrial reactions.165–174 H3PW12O40/SiO2 was chosen by Rafiee et al. as a model system to investigate the effects of different amounts of HPA loading on the support. They proved that in almost all the publications there were differences in catalytic activity among the catalysts with 20, 40 and 60 wt% of H3PW12O40 on silica and sometimes on other supports. Lowering the loading of the deposited H3PW12O40 resulted in a reduction of the catalytic activity. Furthermore, no improvements in the reaction rate and yield were observed by increasing the amount of H3PW12O40 on SiO2 from 40 to 60 wt%, since 40 wt% of H3PW12O40/SiO2 was the best catalyst loading in almost all the cases.175 Acidity measurements of the catalysts by means of potentiometric titration with n-butylamine were carried out. This method enables the determination of the total number of acid sites and their distribution. The addition of H3PW12O40 was accompanied by a gradual increase in both the surface acidity and acid strength up to 40 wt% of H3PW12O40 (Ei = +693 mV), then followed by a decrease in both at a loading of 60 wt% of H3PW12O40. These results indicate that 40 wt% of H3PW12O40/SiO2 contains the strongest acid sites. Here, the increases in the surface area enhance the dispersion of acidic protons up to a monolayer coverage at 40 wt% of H3PW12O40, while a further increase in the HPA content above the monolayer leads to the aggregation of HPA on SiO2 surface, which leads to a decrease in the surface acidity.176 Also, they reported the N2 adsorption–desorption isotherm of H3PW12O40/SiO2. The decrease in the surface area and pore volume from the BET results, i.e. from 311 m2 g−1 and 1.7 cm3 g−1 to 115 m2 g−1 and 0.36 cm3 g−1, respectively, observed for the SiO2 sample in comparison with 40 wt% of H3PW12O40/SiO2 and the relatively low initial adsorption in the isotherm plot can be ascribed to the partial blocking of the micropores by HPA particles.177
They reported that the TGA/DSC profile for 40 wt% H3PW12O40/SiO2 showed three mass losses. The first weight loss was related to the release of the physically adsorbed water molecules. This water release ranged from 34 °C up to 141 °C, with the maximum at about 80 °C. The temperature range, as well as the maximum, could vary according to the amount of water contained initially in the material. A second weight loss in the temperature range of 141–217 °C centred at about 190 °C was ascribed to the dehydration process of the hydrated water molecules. The third step took place in a very wide range of temperature, with two maxima at 233 and 309 °C. The wide range of the third mass loss was related to more than one event under that condition. The global event was attributed to the dehydration of SiO2, the loss of all acidic protons or the beginning of decomposition of the Keggin structure.175
Rafiee et al. prepared nano-silica from rice husk with a high surface area and then fully characterized it.178 It has also been used as a support for HPAs. This form of support also related to the synthesis procedure, showed a higher catalyst loading capacity and higher dispersion of HPA, leading to higher catalytic activity. The catalyst showed excellent activity in some important organic reactions, including the Biginelli, Hantzsch, Mannich and Claisen–Schmidt reactions, together with good reusability. The catalytic activity of this nanocatalyst is an improvement over the commercially available silica used to support H3PW12O40.179 Also the same authors used this support for other HPAs, such as for Mo-containing HPAs and H5CoW12O40, to catalyze other organic reactions.180–183 H5Mo10V2O40 supported on nano-silica (Mo10V2@NSiO2) from rice husk ash was used as a co-catalyst for the C–C coupling reaction as a novel application of HPAs. The authors also developed a convenient catalytic system composed of palladium-deposited poly(N-vinylpyrrolidone) coated-nano zero valent iron (Pd–PVP–Fe) and Mo10V2@NSiO2 nanoparticles as a reusable catalytic system for the Heck coupling reaction under ligand- and base-free conditions.184 However, conventional separation methods may be inefficient for supporting particle sizes below 100 nm. The incorporation of magnetic nanoparticles (MNPs), such as iron oxide, into supports offers a solution to this problem. The strategy of magnetic separation, taking advantage of MNPs, is typically more effective than filtration or centrifugation as it prevents loss of the catalyst. The magnetic separation of MNPs is simple, economical and thus promising for industrial applications. This kind of support is been discussed in the following sections.
G. D. Yadav and co-workers synthesized H3PW12O40 supported on hexagonal mesoporous silica.185 This catalyst was found to be very active and also stable without any deactivation in an environmentally benign route for acetoveratrone synthesis. Different forms of silica show different surface areas and pore sizes and accordingly a different surface acidity. H3PW12O40 was supported on three different carriers by Blasco et al.: a commercial silica, a high surface area amorphous aluminosilicate (MSA) and an all silica-mesoporous MCM-41; and their catalytic properties were determined for the alkylation of 2-butene with isobutene.186 The high surface area of the MCM-41 and MSA supports showed higher acid dispersions compared with SiO2, but H3PW12O40/SiO2 showed the maximum activity, selectivity to trimethylpentane and stability with the time on-stream. The activity of H3PW12O40 depends directly on the interaction of H3PW12O40 with the functional group on the support, and thus, in the H3PW12O40/MSA samples, which have a stronger interaction of H3PW12O40 with the surface sites of the aluminosilicate together with a decrease in both the number of Brönsted acid sites and the average acid strength of those sites of the HPA, lower catalytic activity was predicted. In the H3PW12O40/MCM-41 samples, partial blockage of the monodimensional pores of MCM-41 decreased the accessibility of the reactants to the Brönsted acid sites of the HPA located inside the pores. This pore blockage could be decreased, and the catalytic activity of the H3PW12O40/MCM-41 catalysts increased, by using a MCM-41 sample with a larger pore diameter.
Although, Keggin-type HPAs have been widely studied as homogeneous and heterogeneous catalysts for the oxidation of organic compounds, only a few Wells–Dawson-type polyoxoanions have demonstrated catalytic activity in a heterogeneous form.180 Generally, Keggin structures show more catalytic activity among HPAs,181,182 whereas Wells–Dawson HPAs exhibit greater selectivity and activity in some acid-catalyzed and oxidation reactions.180 The Keggin anions offer limited hydrolytic stability compared to the other types of HPAs, especially when they are supported on interacting substrates. The silica-supported Co(II)-substituted Wells–Dawson HPA salt, Cs6H2P2W17O61Co·H2O, show good activity in an organic solvent. This particular salt not only offers the characteristic acidic and oxidative properties of an HPA skeleton, but also, it exhibits the catalytic activity of the transition metal cation.187
Although impregnation is the widely used method for supporting HPAs, due to the reversible adsorption of HPAs on the supports, HPAs are prone to be dissolved in polar media. Therefore the continuous dissolution of HPAs has become a serious problem leading to some environmental pollution and catalyst waste. In order to overcome the problems of the impregnation method, the sol–gel incorporation method was developed. Here, the silicate structure can trap the HPA molecules, while their acidic property remains to a great extent. Kukovecz et al. compared H3PW12O40–silica composites developed by conventional impregnation and by a sol–gel incorporation technique, and found that the sol–gel derived composites had less dissolution of H3PW12O40, and were more suitable for the purposes of heterogeneous catalysis.188,189
Y. Chen compared the structural and catalytic properties of MCM-41 and SBA-15 as supports for H3PW12O40. The results revealed that the mesoporous materials retained the typical hexagonal mesopores for the support of H3PW12O40. It was found that H3PW12O40 exhibited a higher dispersion within MCM-41 than within SBA-15 and other mesoporous molecular sieves. Moreover, the as-prepared materials were found to be the efficient catalysts for the green synthesis of benzoic acid. In particular, H3PW12O40/MCM-41 exhibited the best catalytic properties due to its suitable textural and structural characteristics.190
A series of HPAs–mesoporous silica composites were prepared from Ni1HPMo12O40 by supporting them on SBA-15 mesoporous silica in different concentrations of the active phase. By impregnating Ni salt on mesoporous silica, the thermal stability of the Keggin structure increases in comparison with its parent bulk HPA. Indeed, the total acidity of the weak and strong acidic sites of Ni1HPMo12O40/SBA-15 composites were obviously increased in comparison with the bulk Ni salt.191
(i) Use of a sacrificial oxidant, such as dioxygen, to scavenge the photogenerated conduction band electrons.
(ii) Adsorbing the TiO2 photocatalyst directly onto the surface of an electrode, where the electrons can be collected by applying a suitable voltage.
(iii) Employing an electron scavenger, such as a POM, to transport electrons from the suspended TiO2 particles.
The first and second solutions are not always efficient, while the third way offers the additional advantage of being able to turn over a larger fraction of the incident photons by using more TiO2 than could be directly adsorbed onto the surface of the anode. The ability of POMs to accept electrons readily has been known for many years. Upon chemical or electrochemical reduction, many POMs form stable, highly coloured mixed-valence species generically termed “heteropoly blues”.192,193 Ozer and Ferry investigated the use of POMs such as H3PW12O40, H4SiW12O40 and H4W10O32 to facilitate the transfer of photogenerated TiO2 conduction band electrons to dioxygen.194 Zhao and co-workers reported similar findings, but also discovered that different product distributions can result when using TiO2 alone or in tandem with POMs.195 POM systems have also been shown to be viable in fuel cell applications. For instance, Dumesic and co-workers demonstrated that it is possible to use H3PMo12O40 to drive a fuel cell by oxidizing CO to CO2, thus bypassing the water gas shift reaction.196 C. Gu, and C. Shannon employed a simple Na3PW12O40 to scavenge electrons from photoexcited TiO2 particles suspended in solution. The reduced POMs were subsequently oxidized back to the parent Keggin complex at the Pt anode, and a 50-fold increase in the methanol oxidation photocurrent was observed as compared to the use of TiO2 alone (Fig. 18).197
Pearson and his co-workers reported the decoration of titania nanotubes synthesized by electrochemical anodization, with gold, silver, platinum and copper nanoparticles, by using H3PW12O40 as a UV-switchable reducing agent with inherent photochemical properties (Fig. 19).198
 | ||
| Fig. 19 Schematic of the formation of TiO2–H3PW12O40 co-catalyst materials decorated with metal nanoparticles. | ||
The formation of certain HPA salts of metal ions (Mn+) of H3PW12O40 show Lewis acidity, originating from the metal cation as an electron pair acceptor, together with the characteristic Brönsted acidity of HPAs, generated from the dissociation of coordinated water under the polarizing effect of the cation. One of these metals is aluminium, which was supported on titania and showed excellent activity.199,200
H3PW12O40 and H4SiW12O40 were deposited on TiO2 by incipient wetness impregnation and used as catalysts for the production of dimethyl ether from methanol. It was found that for HPA loadings ≤2.3 KU nm−2, the HPA remains well dispersed on TiO2, while for higher loadings, 3-dimensional (3D) clusters are formed. At low HPA loadings, an interaction between HPA and the support was observed by the appearance of stronger Lewis acid sites, assigned to coordinately unsaturated Ti4+ species and by the shift in the 1H-NMR spectra of the signal assigned to the protons in the HPA.201
The photocatalytic activity of HPCs in a homogeneous regime has been extensively reported along with the use of supported HPAs in thermal catalysis for acidic and redox processes. However, the nature of the HPAs as highly soluble compounds in polar solvents hinders their use as heterogeneous photocatalysts. Consequently, appropriate heterogenization has been reported by many researchers so that HPAs can be used in heterogeneous reaction media, in particular to carry out the photocatalytic degradation of several pollutants. These reported research studies were reviewed by G. Marcì.202
H3PW12O40 supported on different metal oxides (TiO2, γ-Al2O3, K10 and KSF) and activated carbon were used in the condensation of aniline with ethylacetoacetate to afford the corresponding β-enaminone. The catalytic activity was discussed in relation with the acid strength of the catalysts. The best catalytic activity was obtained with H3PW12O40/TiO2. According to the textural properties of the supports and H3PW12O40/supports data in Rafiee et al.'s report in 2009, in all cases, supported H3PW12O40 showed a higher reactivity compared to the support only on a unit weight basis, except for K10 and KSF montmorillonite, which can mask the catalytic activity of H3PW12O40. Carbon as a support has a high pore volume size so it can entrap HPAs firmly in its pores. The HPA fraction occluded in the pores may interact more weakly than the adsorbed fraction with the reactants, thus leading to the low catalytic activity. Among the supported catalysts, 40 wt% of H3PW12O40/TiO2 was the best in terms of yield and reaction time in β-enaminone synthesis.203 It should be noted that the same authors investigated the effect of this factor in other catalytic reactions with these same kinds of support and proved that it was not similar in all reaction media and depended on the solvents, reactants and mechanism of the organic reactions.
Rafiee and co-workers investigated the acidic properties of H4SiW12O40 on TiO2, SiO2 and alumina. All the supported catalysts had stronger acid sites in comparison with the support only, and according to the mentioned scale, all of them presented very strong acid sites. It was observed that H4SiW12O40/γ-Al2O3 is less acidic than the others. This may be due to the interaction of the strongest protons of H4SiW12O40 with the most basic sites on the alumina surface. This acid–base interaction induces a partial neutralization of the acid sites that leads to a decrease in the acidity and catalytic activity. The acidity of H4SiW12O40/TiO2 is comparable with H4SiW12O40/SiO2.172
There are a number of other new research papers in the literature on the preparation and catalytic activity investigations on HPAs/TiO2.170,173,204–207
A carbon support with a nanorod structure was synthesized from natural potato as a green and very cheap source via a hydrothermal method. A novel type of core/shell nanorod catalyst was synthesized by the immobilization of H3PW12O40 on the surface of the as-prepared C (C@PW). Complete characterization of the core/shell nanorod catalyst was carried out. The C@PW core/shell nanorod was found to be a unique, effective and eco-friendly catalyst for the C–N coupling reactions for a broad range of aryl and alkyl amines and alcohols under aerobic conditions at room temperature. The acidity measurements of C and C@PW by means of potentiometric titration with n-butylamine were used to estimate their relative acid strength. Leaching of the H3PW12O40 from the C was minimized by optimization of the calcination temperature.218
In recent years, considerable progress has been made to synthesize POMs in the nano-size range. Starch microspheres were prepared from low-cost raw materials-soluble starch by reverse-phase microemulsion polymerization methods using phosphorous oxychloride as the linking agent. Si2W18Ti6-loaded starch nanoparticles were prepared by Wang et al.219 Such kinds of support for HPAs were synthesized and characterized by Rafiee et al.220
Researchers working in the fields of catalysis and adsorption have paid much attention to carbon–carbonaceous composite materials. Catalytic filamentous carbons (CFCs) were produced by the decomposition of gaseous hydrocarbons over the iron-group catalysts at 700–900 K.221,222 The morphology and structure of growing CFCs are dictated by the catalyst composition.221,223–226 The adsorption and desorption of H3PW12O40, H6P2W18O62, H6P2W21O71 and H6As2W21O69 on CFCs were studied by M. N. Timofeeva.226 They also studied the influence of the structure of CFCs and their specific surface area on the strength and amount of HPA adsorbed.
In some cases, it is better to modify such kinds of supports to achieve better catalytic activity. As an example, S. K. Bhorodwaj modified the purified clay by refluxing in 4 M HCl acid for various time intervals. The slurry was cooled, filtered and washed thoroughly with water and then dried in an air oven.186,189 The clay samples were then used as a support for HPAs.239
Also, H3PW12O40 supported on dealuminated ultra-stable Y zeolite (DUSY) was prepared by an impregnation method, and then its physico-chemical properties were characterized. The DUSY-supported H3PW12O40 catalyst exhibited high catalytic activity in the liquid phase acetalization of ethylacetoacetate with ethylene glycol for synthesizing fructone. However, continuous leaching of the HPAs into the reaction medium led to a poor catalytic stability. A very high catalytic activity and stability, however, was found on Cs2.5H0.5PW12O40/DUSY catalyst, which could be ascribed to their superacidity, high surface area and water tolerance.240
A facile post-hydrothermal treated process was established for pore size and volume expansion of silica-pillared clay using octadecylamine as the structural agency. The pore size and volume of the starting silica-pillared clay were greatly expanded from 1.9 nm and 0.71 cm3 g−1 to 5.1 nm and 1.21 cm3 g−1, respectively, and the large surface area was successfully conserved. This study provided an effective, controllable approach for pore structure expansion of the silica-pillared clay (Fig. 20).241
 | ||
| Fig. 20 Preparation of silica-pillared clay. Reproduced from ref. 241 with permission from Elsevier. | ||
A new type of nanohybrid material H6P2W18O62/nanoclinoptilolite was fabricated, and performed as an efficient and reusable catalyst in the mild and one-pot condensation of different acetophenones.242 These types of zeolites contain open tetrahedral cages, generating a system of channels, the sizes of which are determined by the content of silicon. These cages are formed with a network of eight-membered and ten-membered rings. The chemical and thermal stability of CP along with its pore system diameter are the most important parameters in studying the conjunction of HPA into the nanozeolite surface.
A series of mesoporous composite frameworks consisting of zirconium oxide and H3PW12O40 and H4SiW12O40 compounds were synthesized in 2014 through a surfactant templating approach. The as-prepared materials possessed ZrO2–H3PW12O40 and ZrO2–H4SiW12O40 frameworks composed of a mesoporous structure, large internal surface area and narrow-sized mesopores. Unlike the specific surface area, the framework composition of these materials played a dominant role in the catalytic performance.250
A comparison of the catalytic activity of H4SiW12O40 or H3PW12O40 supported on ZrO2 calcined at 750 °C and silica-supported H4SiW12O40 calcined at 300 °C with similar loading showed that H4SiW12O40 and H3PW12O40 supported on zirconia acted as efficient and stable solid acid catalysts, whereas H4SiW12O40 supported on silica was leached into the reaction medium and thus catalyzed the reaction homogeneously.251
Starting with the impregnation of a hydrogel-derived zirconyl hydroxide [ZrO(OH)2–CP] with H3PW12O40 in solution, ZrO2 was recently found to be superior to SiO2 as a support material for H3PW12O40 as the high-dispersion of H3PW12O40 on ZrO2 could maintain its Keggin structure at high temperatures up to 750 °C.252 The use of alcogel-derived ZrO(OH)2–AN for the support, compared with conventional hydrogel-derived ZrO(OH)2–CP, produced H3PW12O40/ZrO2 catalysts that showed higher surface areas and better catalytic performance for the selective formation of acrolein from glycerol dehydration. ZrO(OH)2–AN is an anti-sintering ZrO2 nanocrystal prepared by the thermal processing of an alcogel-derived ZrO(OH)2–AN at elevated temperature.253–255
Sometimes, especially in some industrial processes, the reaction conditions affect the stability of the catalyst, for example, in the dehydration of glycerol. This may be due to the high acidity of the catalysts, which is thought to be responsible for coke formation and ultimately the lack of stability observed.256 The effect of acidity on the stability of catalysts for the dehydration of glycerol was studied by grafting zirconia onto silica.256 A comparison of the grafted and ungrafted catalysts showed that they appeared to differ with respect to stability, with the zirconia-grafted catalyst being more stable. This effect was attributed to a minor distortion in the structure of the HPA and its binding strength to the support, leading to a decrease in acidity and, in turn, to an increase in the stability of the catalysts.257 Supported HPAs catalysts are very acidic, typically with a Hammett acidity of ca. 9, and they have been used in the catalytic dehydration of glycerol.258 Different loadings of H4SiW12O40, supported on silica or carbon, have been investigated; however, these catalysts were also found to be unstable. Zirconia-supported HPA catalysts were found to be more thermally stable, with an improved dispersion of the HPA active phase, and this was found to be a key factor in tuning the activity and selectivity towards acrolein.257,258 There are a number of catalytic reactions reported in the literature that were catalyzed by zirconia-supported HPAs.259–263
A bifunctional catalyst with alumina as the support was produced by Liu and co-workers in 2015. They showed that increasing the calcination temperature from 350 to 650 °C can lead to structural evolution of the supported H4SiW12O40 and a subsequent activity change. A large amount of (V, Mo)Ox heteropolyoxo mixed oxides were also formed above 550 °C. However, the Keggin structure of H4SiW12O40 began to dissociate around 450 °C, causing the formation of various WOx species (Fig. 21).265,266
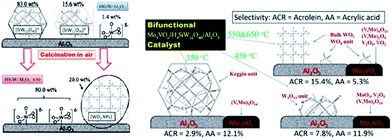 | ||
| Fig. 21 Effect of calcination on the structure of a Keggin unit supported on alumina. Reproduced from ref. 265 and 266 with permission from Elsevier. | ||
Different HPAs containing W, Mo, V, Co, etc. with different hetero atoms supported on alumina were used as catalysts in a number of organic reactions in the literature.164,175,267–273 in these cases, it seems that the Keggin structure is sustained even after catalyst preparation and catalytic reaction.
A magnetite–titania photocatalyst was used as a support for HPAs to make it easy to recover TiO2 powder and HPAs, which commonly require subsequent liquid–solid separation, especially when using the catalyst in water treatment. However, it was found that the magnetite–titania magnetic photocatalyst suffered from serious photodissolution during degradation.283 Researchers have tried adding a silica layer between the magnetic core and photocatalyst to prevent the magnetite core from photodissolution.284,285 An ideal magnetic photocatalyst should possess the following features: (i) high activity in photocatalytic degradation; (ii) no photodissolution during degradation and (iii) a high magnetization for easy magnetic separation. A novel magnetic photocatalyst was prepared by grafting H3PW12O40 anions onto Fe3O4 nanoparticles via a layer of Ag, as shown in Fig. 22.286
The structural and catalytic properties of a Mo-based HPA generated after the impregnation of MoOx onto the surface of porous iron phosphate nanotubes were studied. Nano-sized MoOx–FeP composites with different Mo molar loadings were prepared under acidic conditions.287
Rafiee and Eavani produced a type of magnetically recoverable catalyst by the immobilization of H3PW12O40 on the surface of silica-encapsulated Fe2O3 (Fe2O3@SiO2) nanoparticles. The acidity of the catalyst was measured by potentiometric titration with n-butylamine. The immobilization of H3PW12O40 on Fe2O3@SiO2 was considerably enhanced by the dispersion of acidic protons to 48 meq. per g catalyst. Therefore, a larger fraction of active sites are exposed to the surface, and thus the catalyst exhibited excellent activity in organic reactions, even with low catalyst loading. After the reaction, the catalyst/product separation could be easily achieved with an external magnetic field and more than 95% of the catalyst could usually be recovered. The potentiometric titration curve of the reused catalyst showed that all the acidic sites were preserved during five cycles. According to the ICP-AES results, H3PW12O40 leaching was negligible (Fig. 23).288
In continuation of this work, the preparation, leaching and solid acidity measurements of H3PW12O40, H3PMo12O40 and H4SiW12O40 supported on silica-encapsulated Fe2O3 nanoparticles were investigated. The impregnating solvent and calcination temperature were optimized using various techniques. The best preparation achieved was to impregnate H3PW12O40 on Fe2O3@SiO2 in CH3CN solution, followed by evaporation of the solvent and calcination at 250 °C. The catalysts were mostly spherical in shape and had an average size of approximately 92 nm. Experiments assessing the stability of the supported HPA catalysts indicated their resistance to leaching by methanol as a polar solvent. The acidity of the samples was determined by NH3-TPD and by the chemisorption of pyridine. The strength and dispersion of the protons on H3PW12O40/Fe2O3@SiO2 was significantly high and active surface protons became more available for the reactant.289–292 H3PW12O40-functionalized silica-coated CoFe2O4 nanoparticles were also prepared by Rafiee et al. as a new magnetically separable catalyst.293
A new strategy by a two-component heterogeneous catalytic system composed of Pd–PVP–Fe and magnetically supported H5Mo10V2O40 (Fe@Si–Mo10V2) as a co-catalyst was reported for the Suzuki coupling reaction.294 In order to study the role of Fe@Si–Mo10V2 in the Suzuki coupling reaction, the electron transfer property of Fe@Si–Mo10V2 and Pd–PVP–Fe were investigated by means of cyclic voltammetry measurements. Moreover, this catalytic system could be recovered in a facile manner from the reaction mixture and recycled several times without any significant loss in activity. In this study, the heterogeneity of both components of our catalytic system was investigated and the content of palladium and H5Mo10V2O40 into the filtrates was evaluated quantitatively by ICP-AES. According to the obtained results, a small amount of Pd and H5Mo10V2O40 were leached.292 This magnetically supported catalyst has been used for the extractive oxidative desulfurization of different model oils.295
Also, the synthesis and characterization of a green nanocomposite of H3PW12O40 and starch-coated magnetite nanoparticles in Friedel–Crafts alkylation was reported recently (Fig. 24).296
 | ||
| Fig. 24 H3PW12O40 immobilized on starch-coated MNPs. Reproduced from ref. 296 with permission from Elsevier. | ||
Starch, as a green source of carbon, was used for the preparation of a Fe3O4@carbon core–shell structure for the immobilization of H3PW12O40.220
Another mixed support HPA catalyst was prepared by the wet impregnation of H3PW12O40/TiO2 nanoparticles into SBA-15 and calcined at different temperatures. The characterization results revealed that the textural properties and the acidity of the prepared catalysts could be finely controlled with the simple adjustment of the calcination temperature, and that the structure of the support, decorated with the TiO2 and HPA nanoparticles, was intact even after the modification.303
A series of ZrO2-based hybrid catalysts functionalized by both organosilica groups and Keggin-type HPA, H3PW12O40/ZrO2–Si(Et/Ph)Si and H3PW12O40/ZrO2–Si(Ph) with different structural orderings and pore geometries were controllably prepared by a one-pot template-assisted sol–gel co-condensation–hydrothermal treatment route. Different surface areas from 67 to 353 m2 g−1 were obtained by using different synthesized catalysts. The pore diameters were approximately similar but the surface acidity changed from 779 to 1972 μeq. per g by titration with NaOH.304
These hybrid catalysts have excellent acid catalytic activity, originating from the combination of the strong Brönsted acidity and Lewis acidity, rationally designed porosity and an enhanced surface hydrophobicity. Moreover, ordered 2D hexagonal mesostructured H3PW12O40/ZrO2–Si(Ph)Si-1.0 exhibited a much higher catalytic activity with respect to the 3D interconnected wormhole-like H3PW12O40/ZrO2–Si(Et)Si or H3PW12O40/ZrO2–Si(Ph), which was attributed to the decreased mass transfer limitations of the reactants and products owing to the ordered mesoporous structure (Fig. 25).
Mesoporous-silica–zirconia-supported H3PW12O40 with a regulatable surface acidity was synthesized by an evaporation-induced self-assembly method and used as an oxidative desulfurization catalyst.305 Whereas, mesoporous titania–silica composite was successfully prepared by a modified sol–gel process using the less expensive silica precursor and titanium oxychloride as a titania source. H3PW12O40 was impregnated on the prepared mixed support to produce an acid catalyst.306
In some industrial processes, the catalytic activity of these solid base catalysts are high but it seems difficult to avoid dissolution of these base catalysts. This problem causes an irreversible loss of the catalyst and pollution of the product with an inorganic residue. Using a modified supported catalyst provides the simplest solution to this problem. The catalytic activities of HPAs supported on solid acids such as cation-exchange resins,307,308 sulfated zirconia,309,310 W–Zr–Al–O,310 Zn–Al–O,311 acidic zeolite312,313 and Mn–Ti–O (ref. 314) have been reported. A sulfonated carbon material produced by the treatment of glucose with fuming sulfuric acid gave a high stability,315 and a high activity of HPA-promoted Ta2O5 was also observed.316,317
TiO2 nanoparticles-stabilized H3PW12O40 in SBA-15 were prepared, and a liquid phase hydroamination of α,β-ethylenic compounds with amines was investigated in the presence of this catalyst. The catalysts were prepared by the wet impregnation of H3PW12O40/TiO2 nanoparticles into the SBA-15 and then calcined at different temperatures (Fig. 26).318
 | ||
| Fig. 26 Wet impregnation of H3PW12O40/TiO2 nanoparticles into the SBA-15. Reproduced from ref. 318 with permission from Elsevier. | ||
H3PW12O40/TiO2–SiO2 was synthesized by the impregnation method, which significantly improved the catalytic activity under simulated natural light.319 TiO2–SiO2 was prepared by a sol–gel method, and H3PW12O40/TiO2–SiO2 was synthesized by an impregnation method. The degradation of methyl violet was used as a probe reaction to explore the influencing factors on the photodegradation reaction.
2.4. Encapsulation
The encapsulation of HPAs into porous solid supports with a high surface area, large pore diameter and high pore volume was used as a critical method for improving their catalytic performances and recyclabilities in many heterogeneous catalytic applications. Various porous supports have been applied for the encapsulation of HPAs, including zeolites, mesoporous molecular sieves, metal organic frameworks (MOFs) and mesoporous metal oxides.Mukai et al. synthesized H3PMo12O40 encapsulated in a Y-type zeolite by a “ship in the bottle” synthesis, and the resulting solid catalyst was used in the esterification of acetic acid with ethanol.320 The influences of the Si/Al ratio and the type and amount of counter cations of the zeolite on the amount of encapsulation of H3PMo12O40 molecules were investigated. Y-type zeolites have supercages the diameters of which are approximately 1.3 nm, and these supercages are interconnected by windows 0.74 nm in diameter. It was found that H3PMo12O40 can be formed within the supercages of the support when the number of aluminium atoms per unit cell is roughly in the range of 4–20. When the number exceeds this range, the support is likely to be destroyed during catalyst synthesis, since the durability in acidic solutions is low for supports with high aluminium contents. Moreover, it was difficult to encapsulate H3PMo12O40 using supports with extremely low aluminium contents. These results imply that a moderate number of cation-exchange sites induced by the aluminium atoms included within the support structure are inevitable for the encapsulation of HPA molecules. The role of the counter cations was also investigated using Na+, K+, Cs+, NH4+, Ca2+ and Ba2+. Among these cations, only Cs+ and NH4+ led to a significant amount of H3PMo12O40 encapsulation. When supports with other cations were used, it was impossible or at least difficult to encapsulate H3PMo12O40. The differences among the cations may be attributed to the differences in their abilities to promote the formation of polyanions from MoO42−. When cations such as K+ and Na+ coexist, the formation of polyanions is not observed. Also in the case of divalent cations, MoO42− anions were hardly detected. From these results, it is obvious that cations such as Cs+ and NH4+ have a higher ability to promote polyanion formation than other cations. The amount of polyanion on the support varied from 0.17 g per g of support after the first washing to 0.08 g after the fifth washing, showing that some H3PMo12O40 was lost, probably by decomposition–redeposition phenomena in hot water.
The influences of temperature and solvent addition during synthesis on the amount of HPA encaging and the stability of the resulting encaged catalyst were also investigated by Mukai's group.321 It was found that by adjusting the reaction temperature, the stability of the resulting encaged catalyst could be enhanced to higher levels. Moreover, it was found that by adding t-butyl alcohol to the solution used for the catalyst synthesis, an active and stable encaged catalyst could be prepared, even at low synthesis temperatures. A further improvement of this method was observed by treatment of the modified zeolite with caesium carbonate (Cs–Y zeolite), leading to partially Cs-exchanged salts, which were more stable towards leaching and showed considerable catalytic activity and excellent reusability in the esterification reaction.322
Under microwave irradiation, H3PW12O40 could be successfully synthesized in situ and encapsulated in the supercage of the ultra-stable Y (USY) zeolite in several minutes.323 XPS spectra of the sample confirmed that phosphorous and tungsten in the matrix of USY were combined into Keggin anions. These formed H3PW12O40 could be located in the supercage of the USY (about 13–15 Å in diameter) as the size of a spherical H3PW12O40 molecule is about 11–15 Å (diameter). H3PW12O40 molecules encapsulated in the supercages of USY cannot escape from its smaller apertures of 7.4 Å diameters. Based on the ICP results, the calculated amount of formed H3PW12O40 encapsulated in USY is about 5.6 wt% of the parent USY. This catalyst exhibited high activity in the synthesis of 4,4′-dimethyldiphenylmethane via toluene and formaldehyde and could be utilized as a solid acid catalyst in aqueous solutions.323
Khanchi et al. prepared an HPA–Zr-modified mesoporous silica composite by an incipient wetness method.324 This composite were used for the removal of Sm(III) and Dy(III) ions in aqueous solutions. The adsorption capacity of the composite after four cycles indicated a loss in the adsorption capacity of 7.4% for Sm(III) and 8.2% for Dy(III) metal ions compared to the initial cycle.
The incorporation of HPCs into the pores of a mesoporous material can also be achieved by encapsulating HPCs during the synthesis of the silica material itself. Yang et al. synthesized H3PW12O40 encapsulated in SBA-15 by adding HPA to the SBA-15 synthesis mixture using a sol–gel technique.325 This catalyst was tested in the esterification of acetic acid and showed a higher leaching stability compared with the same impregnated catalyst sample. Toufaily et al. published a similar approach to incorporate H3PW12O40 into MSU.326
Shi et al. obtained SBA-15–encapsulated HPA catalysts by adding P and W (or Mo) sources into the initial sol–gel system during the hydrolysis of tetraethyl orthosilicate to form the Keggin-type HPA in situ.327,328 However, in some cases the HPA loadings were relatively low since higher loadings resulted in a lower catalytic activity due to HPA agglomeration. These catalysts have potential applications in the chemical industry due to their recyclability and reusability.
Martens et al. prepared SBA-15 containing up to 40 wt% of H3PW12O40 via an original direct synthesis method.329 This direct synthesis route was inspired by the proposed supramolecular mechanism of SBA-15 formation. The SBA-15 synthesis is considered to follow a S0H+X−I+ mechanism, in which S0, H+, X− and I+ stand for the tri-block copolymer (EO20PO70EO20), hydronium cations, anions (Cl−) and the positively charged silica source at pH < 2, respectively.
The HPAs were presented at the interface between the silica and polymer. Washing and drying steps did not remove the HPAs since they are trapped inside the SBA-15 particles. In the calcination step, the polymer template decomposes and is eliminated from the pore system but the H3PW12O40 molecules stay fixed onto the pore walls. The original direct synthesis method were also used for the synthesis of MCM-41 incorporated with transition metal-substituted HPAs (PW11O39M15−, M1 = Ni2+, Co2+ or Cu2+)330 The samples exhibited excellent catalytic performance for the esterification of n-butanol with acetic acid, which was better than that of the supported lacunary POM PW11O397− and the impregnated samples.
Chen et al. used a one-pot hydrothermal method for the preparation of SBA-15-encapsulated H3PW12O40.331 This catalyst showed the highest photocatalytic efficiency for the degradation of rhodamine-B and also the highest microwave catalytic efficiency in the synthesis of isoamyl acetate. The reusability of the catalyst was also investigated. It was found that 20 wt% of H3PW12O40/SBA-15 showed excellent reusability after three cycles, but 40 wt% of H3PW12O40/SBA-15 was not reusable.
In another way, the support surfaces were first functionalized with an example amine ligand and then treated with HPAs, which is categorized as a tethering method (discussed in Section 2.5).
One possible route for the encapsulation of HPAs onto a MOF framework is the introduction of HPA during the synthesis, i.e. in an in situ production. A lot of new structures could be obtained this way, depending on the type of HPA, metal ion and organic linker. However, in some cases, the obtained catalysts are not reusable or thermally stable.
Liu et al. reported the syntheses of a number of remarkable crystalline compounds [Cu2(BTC)4/3(H2O)2]6[HnXM12O40]·(C4H12N)2 (X = Si, Ge, P, As; M = W, Mo) the using simple one-step hydrothermal reaction of copper nitrate, benzentricaboxylate (BTC) and different Keggin HPAs.332 In these compounds, the catalytically active HPAs were alternately arrayed as non-coordinating guests in the cages of a MOF host matrix. This system is stable until ca. 250 °C and no HPA leaching or framework decomposition was observed in this study. The catalytic activity of a compound containing the H3PW12O40 species was examined through the hydrolysis of esters in excess water, which showed a high catalytic activity and that the catalyst could be used repeatedly without activity loss.
An original synthesis approach to prepare Cu3(BTC)2 (BTC = benzene tricarboxylic acid)-encapsulated H3PW12O40 involving mixing the reagents at room temperature, followed by quenching in liquid nitrogen and freeze drying was reported by Martens' group.333 The sample was isostructural in the HKUST-1 framework, accommodating H3PW12O40 ions in the cavities. The catalytic properties of this catalyst were assessed using a model esterification reaction of acetic acid with 1-propanol. The catalyst was partially dissolved in the presence of acetic acid. At a high molar ratio of acetic acid to 1-propanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), a leaching of Cu2+ and H3PW12O40 was observed. However, at a low molar ratio of acetic acid to 1-propanol (1
2), a leaching of Cu2+ and H3PW12O40 was observed. However, at a low molar ratio of acetic acid to 1-propanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40), the leaching of Cu2+ and H3PW12O40 could be prevented and the catalyst was stable. However, the BET surface area and catalytic activity was significantly decreased after several cycles.333
40), the leaching of Cu2+ and H3PW12O40 could be prevented and the catalyst was stable. However, the BET surface area and catalytic activity was significantly decreased after several cycles.333
Rafiee and Nobakht reported the use of Cu3(BTC)2-encapsulated Keggin-type HPAs (including H3PW12O40, H3PMo12O40 and H4SiW12O40) for the selective oxidation of sulfides and for the deep desulfurization of model fuels.334,335 It was observed that the catalytic system could be recycled at least four times with little decrease in catalytic activity.
Hill et al. used a combination of Keggin-type [CuPW11O39]5− and MOF-199 in air-based oxidations.336 This sample catalyzed the rapid chemo- and shape-selective oxidation of thiols to disulfides and, more significantly, the rapid and sustained removal of toxic H2S via the following reaction:
| H2S + ½O2 (air) → ⅛S8 + H2O |
The hydrolytic stability of the catalyst was greater than that of either the MOF or the HPA alone. The strong HPA–MOF interactions dramatically increased the rate of the air-based oxidations catalyzed by the HPA guest.336
H6PMo9V3@Cu3(BTC)2 was used as catalyst in the liquid hydroxylation of benzene with O2.337 In only 20 min, a high TOF (44.2 h−1) was obtained. No significant decrease in phenol yield was observed for the recycling experiments. During the reaction, the liberation of a fraction of HPAs could lead to a slight deactivation of the system. From the powder XRD patterns, it was found that the crystallinity of the recovered catalyst was changed a little in the three recycles.337
Gao et al. presented a new hybrid material, i.e. HPA@MOF@SBA-15, in which metal–organic MOF-199-encapsulated HPA catalyst was facilely confined in mesoporous SBA-15.338 The organic bridged inside liner was stabilized by coating with an outer inorganic rigid SBA-15, allowing the catalyst to exhibit exceptional stabilities in the liquid oxidation reaction. These results confirmed a new method to stabilize the functionalized MOF structure via encapsulating it in SBA-15, which is also a novel strategy for synthesizing micropore mesoporous hybrid materials.
Duan et al. reported the preparation of heterogeneous asymmetric catalysts by incorporating the [BW12O40]5− and the chiral group, i.e. L- or D-pyrrolidin-2-ylimidazole (PYI), into one single framework (Fig. 27).339 This catalyst was used in the asymmetric dihydroxylation of aryl olefins. Excellent stereoselectivity was observed due to the coexistence of both the chiral directors and the oxidants within a confined space. The catalysts could be reused at least three times with only a moderate loss of activity (from 75% to 68% of the yield) and a slight decrease in the selectivity (ee values from >95% to90%).
 | ||
| Fig. 27 Asymmetric POM–MOF catalyst.339 Reprinted with permission from J. Am. Chem. Soc., 2013, 135, 10186. Copyright © 2013 American Chemical Society. | ||
Recently, a new strategy was proposed to construct the IL, HPA and MOF composite.340 The [SO3H–(CH2)3–HIM]3PW12O40 was encapsulated within the framework of MIL-100(Fe). It demonstrated a significant increase in acidity and had both Brönsted and Lewis acid sites, compared with the HPA-based MOF. This composite was used as a catalyst for the esterification of oleic acid with ethanol. The catalyst could be easily recovered and reused six times, with no obvious leaching of the active component.
The encapsulation of HPA into the pores of MOF can also be achieved by its adsorption on a preformed MOF. This strategy was developed after the discovery of MIL-101 by Férey and co-workers.341 The zeotype cavities of two different sizes, the fully accessible porosity and therefore the outstanding sorption properties, together with a high thermal and chemical stability, make MIL-101 an excellent candidate for supporting HPA species. Various groups have reported the encapsulation of Keggin- and Dawson-type HPAs onto MIL-101 and the use of the resulting materials in heterogeneous catalysis.342–352
The sizes of the Keggin HPAs (13–14 Å diameters) are smaller than the pentagonal window openings of the large and medium-sized cages of MIL-101, and also bigger than the hexagonal window openings of the large cages. In the MIL-101 encapsulated HPA composites, the HPA guests are only encapsulated into the large cavities using the impregnation approach. Thus, the medium-sized cages, which represent 2/3 of the total number of cavities of MIL-101, remain unfilled (Fig. 28).
It is obvious that the encapsulation of such active species into the medium cavities would offer many advantages, like a better dispersion and utilization of the support. Moreover, if contained in the medium cavities, leaching of the HPAs should never be a problem, since they are bigger than the windows of these cavities. In order to achieve highly dispersed and reusable catalysts, the successful one-pot encapsulation of HPAs in the large and medium mesoporous cavities of MIL-101 (also known as ‘‘bottle around the ship’’ or ‘‘templated synthesis’’) have been explored by various research groups.353,354 It has been shown that these materials are stable to HPA leaching and can be used repeatedly without any considerable loss of activity or selectivity.
Recently, Ji et al. reported the Friedel–Crafts acylation of anisole with benzoyl chloride over H3PW12O40 encapsulated into a zeolite imidazolate framework.355 This novel subclass of MOFs has the advantages of both zeolite and conventional MOFs. It was found that 14.6–31.7 wt% of H3PW12O40 were encapsulated into the zeolite imidazolate framework. This catalyst was recycled and reused five times without any appreciable loss of activity or selectivity.
Wells–Dawson POM-based MOFs were prepared recently by two research groups.356,357 These catalysts showed remarkable photocatalytic and electrocatalytic activities in aqueous solutions and could be reused several times.
Peng et al. reported the encapsulation of Cs salt of HPAs, CsxH3−xPMo12O40 and CsyH5−yPMo10V2O40 in silica and the use of the resulting materials in the liquid phase oxidation of benzyl alcohol to benzaldehyde.358 The catalytic activities were strongly dependent on the H+/Cs+ ratio in the HPAs. The best results were obtained for a value of one, which indicated that not only oxidative properties but also acidity was necessary to achieve a good catalyst. The catalyst could be recycled up to five times without loss of activity, and no leaching of the HPA was observed in the solution.
Wang et al. described the synthesis of XW11On−39 (X = P, Si, Ge, B) encapsulated in silica.359–361 These materials were used in the photocatalytic degradation of malic and formic acids and displayed interesting properties, with no leaching of the HPAs.
Mesoporous-silica-pillared clay materials with different contents of H3PW12O40 were synthesized by introducing HPA into a clay interlayer template in an acidic suspension using a sol–gel method.362 The catalysts showed a homogeneous dispersion of the H3PW12O40 molecules, even at 25 wt% loading. The encapsulated materials exhibited better catalytic performance than the impregnated samples in the oxidative desulfurization of dibenzothiophene-containing model oil.
Dou and Zeng reported the targeted synthesis of H4SiMo12O40 within mesoporous silica hollow spheres.363 The mesoporous shell was formed by removal of the organic template using thermal treatment of as-synthesized MoO2@SiO2 core–shell spheres. The encapsulated MoO2 was oxidized to Mo6+ and infused into the mesoporous silica shells, forming heptamolybdate species (Mo7O246−) uniformly dispersed on the mesopore surfaces of silica. After hydration with water, H4SiMo12O40 was formed by reaction between the surface Mo7O246− and silica species. The prepared H4SiMo12O40@mSiO2 hollow spheres were tested for the Friedel–Crafts alkylation of toluene by benzyl alcohol. This catalyst showed excellent catalytic activity and could be reused after regeneration.
Okada et al. developed a highly active and water-tolerant solid acid catalyst by encapsulating HPA into hydrophobic hollow polymethylsiloxane microspherical particles.364 H3PW12O40 and Cs2.5H0.5PW12O40 were used for this purpose. Their synthesis approach was based on the deposition of an organosilica shell on water droplets in a water-in-oil (W/O) emulsion through a sol–gel reaction of alkylsilyl trichlorides. Droplets of an aqueous solution of H3PW12O40 or Cs2.5H0.5PW12O40 suspension were dispersed in viscous liquid paraffin to create a W/O emulsion. Methyltrichlorosilane was subsequently hydrolyzed on the droplets to form Cs2.5H0.5PW12O40–polymethylsilane microcapsules. As a result of water vapourization, fine particles of HPCs were successfully immobilized into the capsules. These catalysts were active in the hydrolysis of ethyl acetate in a large excess of water. The activity of the Cs2.5H0.5PW12O40-encapsulated catalyst was maintained, and it showed low leaching. The polymethylsiloxane shell plays several roles, including the shielding of Cs2.5H0.5PW12O40 and allowing penetration of the reactants (ethyl acetate and water) and products (ethanol and acetic acid) through the microporous membrane.
Titania-encapsulated HPAs were also synthesized with a high surface area and interesting photocatalytic properties. Titanium tetraisopropoxide was used as the TiO2 source.365,366
Guo et al. developed the synthesis of ordered mesoporous PW–TiO2 composites by a block copolymer surfactant-assisted templating route.367–369 These solids were used for the photocatalytic degradation of various substrates, such as diethyl phthalate, methyl orange, crystal violet, rhodamine or methylene blue.
Pizzio et al. described the synthesis of HPA–TiO2 mesoporous nanocomposites by a method derived from that of Guo et al., but with urea as the pore forming agent.370–373 They suggested that the interaction between HPA and TiO2 was probably ionic before calcination and then became covalent after calcination at higher temperatures.
Guo et al. reported two efficient heterogeneous photocatalysts, i.e. H3PW12O40/Ta2O5 and H6P2W18O62/Ta2O5, with an amorphous phase, microporosities or micro-mesoporosities, a narrow band gap and nanometre sizes.374 These catalysts were prepared by a one-step sol–gel process following hydrothermal treatment at a constant heating rate. H3PW12O40/Ta2O5 and H6P2W18O62/Ta2O5 were successfully used in the visible-light photocatalytic degradation of salicylic acid and rhodamine-B. These catalysts were easily separated and recovered, and little deactivation of the catalysts was observed after three catalytic cycles. Guo's group also synthesized the H3PW12O40/Ta2O5 catalyst with the addition of a tri-block copolymer surfactant as the template.375 The catalytic performance of the sample was evaluated in the esterification of lauric acid and myristic acid and in the transesterification of tripalmitin as well as in the direct use of soybean oil for biodiesel production. Regardless of the presence of free fatty acids, the H3PW12O40/Ta2O5 composite showed high reactivity and selectivity towards simultaneous esterification and transesterification under mild conditions. The catalyst could be recovered, reactivated and reused several times.375
Up to now, there have only been a few reports of the synthesis of HPA entrapped within ZrO2. In them, zirconium n-butoxide was used as the ZrO2 source and the samples were used for photocatalysis.376–380 As with the other metal oxides, HPA@ZrO2 systems are fully recyclable.
Armatas et al. described the synthesis and catalytic activity of HPAs entrapped within mesoporous Co3O4 (ref. 381) and Cr2O3.382 Co3O4/H3PW12O40 composites were synthesized using the hard templating method. Cubic mesoporous KIT-6 silica was used as a template. A series of mesoporous materials with different HPA loading was obtained after removing the silica by treatment with HF. The products had a 3D cubic structure with a large internal surface area and narrow distribution of pore sizes, and exhibited outstanding catalytic activity in the direct decomposition of N2O.381
Mesoporous nanocomposite frameworks of Cr2O3 and H3PMo12O40 compounds were prepared via a “nanocasting” method, using mesoporous silica SBA-15 as the template. They demonstrated for the first time, the feasibility of the proposed synthesis method to achieve a high loading of HPA clusters in mesoporous metal oxide frameworks. These materials, with up to 63 wt% of HPA content, retained the crystal structure of the silica template. The composites also showed excellent catalytic activity and stability in the oxidation of 1-phenylethanol using H2O2 as the oxidant.382
2.5. Tethering
In the tethering method, HPA is attached to the support surface via a spacer species. Amino-functionalization is one of the most commonly used modifications, and is often realized by using silane coupling agents, with amino-alkyl silane derivatives used in the tethering method. The interaction between the NH2 groups in the silanes and the HPA molecules has been demonstrated to be based on first the acid–base interaction between the –NH2 groups and HPA molecules, and second the interaction between the NH2 groups and the interior water molecules inside the HPA:383Nowadays, the functionalization of mesoporous silica has started to attract particular interest, mainly due to potential applications of these materials in catalysis, drug delivery and adsorption.384–387 Mesoporous supports became more versatile after the development of procedures to functionalize the surfaces or framework and could be elevated to the next level of utility by surface functionalization. This effort can help fine tune the interactions with guest molecules and optimize the bulk and interfacial properties of the materials. As an example, the outer mesoporous silica shell of the core–shell magnetic mesoporous silica nanocomposite makes it possible for multifunctional surface modification. 3-Aminopropy(l)triethoxysilane (APTES) is generally used to generate terminal amino groups (ANH2) and form covalent bonds with functional groups on the surface of the modified material. Up to now, much work has been done to incorporate amino groups on the surface of mesoporous silicas, such as HSM, MCM-41, SBA-15 and so forth.388–392 In 2013, Zhao and co-workers successfully anchored H3PW12O40 to the surface of amino-functionalized Fe3O4@SiO2@meso-SiO2 microspheres by means of chemical bonding to the aminosilane groups, with an aim to remove unwanted organic compounds from aqueous media.393
Transition metal-substituted POM of the type [MII(H2O)PW11O39]5− (M = Co, Zn) and [SiW9O37{CoII(H2O)}3]10− have been chemically anchored to modified macroporous (400 nm pores), mesoporous (2.8 nm pores) and amorphous silica surfaces by B. J. S. Johnson (Fig. 29).394
Two types of POM-functionalized magnetic nanoparticles catalysts, consisting of H3PW12O40 supported on surface-modified Fe3O4 MNPs, were prepared using an easy synthetic route (Fig. 30).395
 | ||
| Fig. 30 Synthesis pathway for the preparation of H3PW12O40 supported on surface-modified Fe3O4 MNPs. | ||
It has been shown that the presence of water in the atmosphere is necessary for the hydrolysis of silane molecules and is also essential for silanization modification of the silica particles. Efficient silanization modification has enabled the high loading ratio of HPA onto the surface of the silica.396
Palygorskite (also known as attapulgite), a hydrated magnesium aluminium silicate with the theoretical formula of (Mg,Al)4(Si)8(O,OH,H2O)26·nH2O commonly has a lath or fibrous morphology and is rich in reactive surface OH groups. It is characterized by its parallel pores.397–401 The above-mentioned spacer arm methodology has also been attempted in modifying palygorskite, in particular, with APTES (Fig. 31).402–406
Different kinds of magnetic POMs were obtained by a simple sonication between functionalized magnetic nanoparticles and POM. These materials could be used not only as a highly active acid catalyst, but also as a catalyst support for chiral amines, with one of them presented in Fig. 32 as an example.407
 | ||
| Fig. 32 Magnetic nanoparticle functionalized by a chiral amine. Reproduced from ref. 407 with permission from the Royal Society of Chemistry. | ||
One heterogenization technique is known as supported liquid-aqueous phase catalysis, wherein catalysts, dissolved in solvents, are strongly adsorbed onto supports, such as silica. In such systems, considering the liquid state of the catalyst-containing phase, contact with a reactant in a fluid phase is maximized. Typically, hydrophilic solvents, such as water, ethylene glycol and even polyethylene oxide, have been used. In a new system, a solvent, polyethylene and/or propylene oxide was covalently anchored to a silica surface. This tethering of the polyethylene and/or propylene oxide onto the silica supports has the perceived additional advantages of enabling the anchoring of both hydrophilic and hydrophobic solvents, and of preventing leaching of the solvent phase and the associated catalyst from the heterogeneous support.408
HPAs could also be supported on these functionalized surface area, as shown in Fig. 33.
 | ||
| Fig. 33 Imobilization of HPAs in tethered silica. Reproduced from ref. 408 with permission from Elsevier. | ||
A new inorganic–organic nanohybrid material H4SiW12O40/pyridino-MCM-41 was prepared, and performed as an efficient, eco-friendly and highly recyclable catalyst for the one-pot multi-component synthesis of different substituted 1-amidoalkyl-2-naphthols under solvent-free conditions. The nanohybrid catalyst was prepared through electrostatic anchoring of Keggin H4SiW12O40 on the surface of MCM-41 nanoparticles modified by N-[3-(triethoxysilyl)propyl]isonicotinamide.409
3-Mercaptopropyl-triethoxysilane, with a terminal thiol group (–SH), a grafting method in which the hydroxyl group of the mesoporous silica reacts with the organic silane to form a functional group on the surface layer of mesoporous silica by covalent bonding, is one of the most promising methods for the surface functionalization of mesoporous silica. Mesostructured cellular foam (MCF) silica was prepared via a surfactant templating method. The MCF silica was then modified by grafting APTES to create a positive charge on the surface, and thus, to provide sites for the immobilization of H3PMo12O40 (Fig. 34).410
2.6. Grafting
Grafting refers to the covalent attachment of HPA to the support surface. Although, this method is a very promising approach for the heterogenization of HPA catalysts, only a few examples of covalent linking have been reported so far. This is due to the fact that achieving covalent immobilization requires carefully designed anchors and a surface modification method to simplify the preparation steps to avoid high costs and to extend the large-scale application potential.Errington's group reported the use of mono-functional alkoxide HPA derivatives, [(RO)MW5O18]n− (M = Ti, Zr, Nb), for the covalent attachment of TiW5O184− to alkanol-derivatized silicon surfaces using a stepwise method (Fig. 35).411 Here, TiW5O184− clusters were attached to Si through covalent Ti–O–C bonds by alcoholysis of the MeO–Ti bond with a preassembled alkanol monolayer on silicon surfaces.
Rao et al. synthesized a zirconium phosphate supported HPA catalyst by a surface grafting method (Fig. 36).412 A series of HPA catalysts with different W loadings (1–25 wt%) were prepared and successfully used in the esterification of palmitic acid with methanol. These catalysts were resistant to leaching of the active HPA, were readily recovered and could be recycled without major activity loss.
Yang et al. reported novel HPA-functionalized mesoporous hybrid silica by the formation of Si–O–W covalent bonds.413 A hexagonal mesostructure was synthesized directly with TEOS and Keggin-type SiW11O398− (SiW11) as precursors in the presence of EO20PO70EO20 (P123) as a block copolymer. SiW11 reacts with TEOS to yield free SiW11Si2 molecules at step (I); then SiW11Si2 penetrates into the preorganized silica framework and reacts with the framework by condensation between SiOH groups on both surfaces to block the terminal of silica at step (II). Thus, perfect Keggin units can be covalently linked on the walls of mesoporous silica after removing the template (Fig. 37). The HPA concentration in the initial mixture, the prehydrolysis time of TEOS and the temperature for ageing are important factors influencing the composition and structure of the hybrid material.
Tour et al. reported the direct covalent grafting of HPA onto silica surfaces using diazonium chemistry.414 A diazonium salt-derived organoimido hexamolybdate was synthesized and covalently immobilized on silica surfaces to form both monolayers and multilayers.
Recent progress in “surface organometallic chemistry” has opened a new route to HPC-based materials.415,416 These strategies constitute an alternative method for the covalent grafting compared to the classical immobilization techniques of HPAs involving sol–gel and classical impregnation techniques. The principle consists in using: (i) a partially dehydroxylated silica support bearing the desired functionality and (ii) the appropriate dehydrated HPA corresponding to this functionality. Thus, it is possible to carefully control the distribution of HPA units on the silica support and to prevent its leaching in polar solvents. Lefebvre et al. used this strategy with dehydrated H4PVMo11O40 on silica modified by silane surface groups.415 The use of silica modified by SiH surface groups led indeed to HPA covalently linked to the oxide support. Moreover, the use of partially dehydroxylated silica led to control of the HPA loading. Therefore, this approach is a useful way to obtain well-defined and well-distributed HPA surface species and control of the HPA surface interaction.417
The heterogenization of HPA catalysts by direct covalent immobilization in polymer matrices has also been reported. Wang et al. reported a simple and effective strategy for covalently immobilizing Wells–Dawson-type HPCs onto a solid macroporous resin.418 The channel surface of the resin was post-functionalized with alkynyl groups. Then, organic-modified HPA clusters were “clicked” onto the functionalized channel surface of the resin to efficiently generate a solid catalyst (Fig. 38). The catalytic performance of this solid was examined in tetrahydrothiophene oxidation. The solid catalyst was found to be efficient and had a high selectivity. Moreover, it could be reused several times without detectable catalytic activity loss.
Yin and Liu used a new strategy based on the emulsion polymerization technique to synthesize HPA-latex material. A trivacant Keggin-type HPA, i.e. Na9PW9O34, was functionalized with four short organic tails with methyl methacrylate (MMA) as the ending groups. A target hybrid molecule, K3PW9O37(SiC7H11O2)4, was used as the surfactant in a typical emulsion polymerization reaction of styrene. Due to the copolymerization of MMA and styrene, the HPA clusters were chemically bound onto the surface of the cross-linked polystyrene latex particles because of the multiple covalent linkages after the polymerization reaction. The obtained latex demonstrated a typical core–shell structure with a polystyrene core and an HPAs close-packed shell.419 The HPA-latex could be re-collected from the reaction solution through centrifugation or other convenient technologies. The system could be used for the purpose of “quasi-homogeneous catalysis”, which is a promising method for bridging homogeneous and heterogeneous catalysis.
3. Comparisons and the prospects of the heterogenization methods
A number of reasons justify the need for heterogenization of HPCs, one major example being for recovery and recycling of the catalyst. A correlation diagram between the various heterogenization methods and recycling of the HPC catalysts is shown in Fig. 1 and discussed in Section 2. However, as can be seen in Sections 2.1–2.6, numerous examples of heterogenized HPCs have been reported in the literature, and the viable options are too wide to allow the proposal of a comprehensive set of rules. It is thus suggested that the best heterogenization method must be selected for each individual case, considering the structure of the catalyst, the types of reactions, the method of catalyst recovery and recycling, the physical and chemical properties of the products and if any there is by-product, etc. Still, some general considerations can be offered by applying common sense and from considering the literature results.In a general view, the heterogenization method could be related with the interaction between the HPCs and the supports. Therefore, there are two typical kinds of heterogenized HPCs: supported HPCs (dispersion, encapsulation, tethering and grafting methods) and HPC salts (precipitation and hybridization methods). However, several matters should be considered when choosing the best methods for the heterogenization of HPCs.
In supported HPCs, the choice of the support itself is likely the most relevant. For many catalytic applications, the dispersion of HPC on a high surface area carrier is desirable. The support can either play only a mechanical role or it can be used to modify the catalytic properties of the HPC deposited on the surface by favouring the growth of certain structures or by inducing different types of interaction.
In the dispersion method, the catalytic activity and leaching stability of HPC are affected by its interaction with the support surface and monolayer coverage capacity of the support. Rafiee et al. compared the acidity, activity, leaching and recyclability of H3PW12O40/aerosol silica and H3PW12O40/Fe2O3@SiO2 catalysts in the synthesis of biologically active compounds in water.291 With similar POM loading, the H3PW12O40/Fe2O3@SiO2 catalyst showed a higher dispersion of acid sites and a higher catalytic activity compared with H3PW12O40/aerosol silica. This is due to the high monolayer coverage capacity of the Fe2O3@SiO2 support. H3PW12O40/aerosol silica showed poor reusability, and a significant loss of catalytic activity was observed after the first run. However, the H3PW12O40/Fe2O3@SiO2 catalyst could be recovered and reused four times. About 27.3% of the initial POM content though leached into the reaction mixture during four successive runs but the yield of the product was not substantially changed (10% difference). Kholdeeva et al. reported the immobilization of several redox active HPCs on two commercial active carbons: microporous L2701 and a mesoporous Sibunit. The interaction between HPC and carbon appeared to be so strong that even after washing with hot ethanol or acetic acid, the HPC loading remained unchanged. Depending on the nature of the HPC and carbon, irreversible immobilization of 7–17 wt% of HPC occurred.420 However, the activity of carbon-supported HPCs was lower than that of silica-supported HPCs in α-pinene and isobutyraldehyde co-oxidation.
Generally, despite a few cases, in the dispersion method, the support does not contain specific anchoring sites for dispersed HPC particles thus, the leaching of HPC species usually occurs into polar media.
Encapsulation of the HPCs into a porous matrix is one way to increase the leaching stability. But the catalysts tolerance for structurally different substrates is affected by the encapsulation, since the reagents may not be free to interact with the HPC into the pores. For example, Liu et al. synthesized [Cu12(BTC)8][H3PW12O40] as a highly stable crystalline catalyst. This catalyst was used in the hydrolysis of ester in excess water.332 It was found that the catalytic activity and selectivity depended on the size and accessibility of the substrates for the surface pores. The conversion of methyl acetate and ethyl acetate molecules with dimensions of 4.87 × 3.08 Å and 6.11 × 3.11 Å, respectively, reached ∼64% after 5 h. In contrast, the conversion of ethyl benzoate with molecular dimensions of 8.96 × 4.65 Å was reduced to below 20% under similar conditions. The conversion of 4-methyl-phenyl propionate, a larger ester with dimensions of 10.61 × 4.04 Å, was still below 1% after 24 h.
The immobilization of POMs on specially modified supports via the formation of a chemical bond (tethering and grafting methods) is another way to improve the leaching stability of the catalyst. Mahjoub et al. compared the activity and stability of H3PW12O40 supported on SBA-15 (SBA/W), H3PW12O40 and the hexamethylphosphoramide (HMPA) hybrid, H3PW12O40. n[C6H18N3OP]NH2, supported on SBA-15 (SBA/HMPAW) and amine, functionalized SBA-15 ([SBA/NH2]/HMPAW) in the epoxidation of olefins in CH2Cl2 as the solvent.421 In the SBA/HMPAW catalyst, the acidic protons of the H3PW12O40 were engaged in hydrogen bonding with the HMPA. Hence, the acidity and catalytic activity were reduced significantly compared with SBA/W. In the ([SBA/NH2]/HMPAW) catalyst, amine functionalization improved the sorption properties and guest incorporation within the SBA-15 mesoporous host compared to the non-functionalized SBA-15. Thus, a higher epoxides yield is achieved with the amine functionalized systems ([SBA/NH2]/HMPAW). Despite the lower catalytic activity, the [SBA/NH2]/HMPAW catalyst was heterogeneous and reusable and could be recovered and used for at least four runs without any significant loss in activity. Similar results were obtained by Hu's group,422 who synthesized a series of novel water-tolerant POM-containing composite photocatalysts and investigated their activities and stabilities in the photocatalytic degradation of parathion-methyl. As for the stability of the POM on the supporters, selecting lacunary or substituted POM as the inorganic precursors is better than the saturated POM. Therefore, the strong chemical interactions between the lacunary or substituted POM unit and the matrix (i.e. via formation of a covalent or coordination bond) would effectively avoid the leakage of the active component from the matrix. However, the photocatalytic activity of the saturated POM is somewhat higher than that of the lacunary or substituted one.422
As a result of the tethering and grafting methods, a decrease in chemical activity should be expected due to the interaction of the linker with POM, but exceptions have been reported. For example, the photocatalytic activities of K6[Ni(H2O)SiW11O39] supported on amine-modified silica materials (SiW11Ni/NH2/SiO2) have been improved compared with the K6[Ni(H2O)SiW11O39] in homogeneous systems due to their porous structures.423 In addition, the leaching of POM from the support was controlled effectively due to the chemical attachment of the cluster onto the support. Therefore, the judgement about where on the catalyst the handle for the connection to the support should be placed, and what kind of linker (tethering and grafting methods) should be inserted between the catalyst active site and the support are two important factors that should be considered. The catalytic activity and stability are also related to the types of interaction of the HPCs with the linker (covalent and non-covalent interactions).
Among various solid supports, silica can be utilized in different heterogenization methods, including dispersion, encapsulation, tethering and grafting. Since Keggin-type H3PW12O40 was the first commercialized in the industrial research field, a large number of heterogeneous catalysts have been synthesized by the immobilization and/or incorporation of H3PW12O40 on/in the silica matrix. The Keggin ion interacts with the surface hydroxyl groups of the silica by an exchange reaction, leading to the formation of water and the replacement of the surface hydroxyl anion by a Keggin anion:
Most of the HPAs easily interact with the basic SiOH groups on the silica matrix. In the case of the nanoparticles, however, more HPA molecules might be exposed to the outer surface of the silica nanoparticles due to the high surface-to-volume ratio of nanoparticles. This issue has been investigated by various research groups.288,291,424 The reduction of particle size of the catalyst improves the surface of the nanoparticle. As the particle size of silica is decreased, the exposure of H3PW12O40 was increased and thus the amount of surface acid sites was also increased. Hence, the catalytic activity of H3PW12O40/SiO2 nanoparticles was higher than the activities of pure H3PW12O40 and bulk H3PW12O40/SiO2 catalysts.424
Silica-encapsulated H3PW12O40 was prepared simply via a sol–gel process. This catalyst was used as a green and recyclable photocatalyst, under O2 gas as the sole catalyst reoxidant and in acetonitrile as the polar solvent. The catalyst was reused several times without a significant decrease in the yield or reaction rate.425 However, a substantial decrease in the activity and selectivity of the encapsulated HPA may be observed due to the heterogeneous nature of the support materials in specific reaction media, and due to steric and diffusion factors. Hollow nano-structured silica materials are considered as promising nano-supports for the encapsulation of HPAs. In this regard, void spaces within mesoporous silica spheres can function as nano-reactors for catalytic reaction. Compared to mesoporous bulk silica, hollow spheres have shorter pore channels and thus experience less travelling blockage, which is highly desirable for the mass transfer of reactants and products, especially for liquid phase reactions.363
H3PW12O40 was tethered on the inner surface of mesoporous MCM-41, fume silica and silica-gel by means of chemical bonding to aminosilane groups.426 MCM-41 showed the largest amine to silica ratio due to its large surface area and the large number of available surface hydroxyl groups. In contrast, functionalized MCM-41 showed the least tendency for anchoring with HPAs in comparison with the functionalized fume silica with a lower surface area. This was due to the smaller accessibility of its inner surface, which is mostly confined within the channels. For all the samples, the strong interaction of H3PW12O40 with amine groups results in the highest HPA retention on the silica surface when used as a catalyst in polar media.
The covalent attachment of H3PW12O40 onto the silica surface via a grafting method had not been reported, until now. This is due to the fact that covalent immobilization requires suitable anchors in HPA structure and surface modification of the support. These anchoring sites are provided via removal of one or more M atoms to form “lacunary Keggin molecules” or by replacement of one or more addenda atoms and the terminal oxo-group by a suitable metal atom cation to form metal-substituted polyanions.
Generally, in dispersion, encapsulation, tethering and grafting methods, the choice of the support is crucial, because the properties of the support influence the catalyst behaviour at every level. Relevant features of the support are: its cost, commercial availability and degree of functionalization. Each of these factors should be considered in the design of the catalyst and when choosing the method of heterogenization.
The solubility properties are the other important factors that should be considered in the choice of a suitable counter cation for HPCs (precipitation and hybridization methods). There is no general rule about whether working with a soluble catalyst is better or worse than working with an insoluble one. In principle, one can expect a heterogeneous catalyst to be less reactive than a homogeneous one, but a number of results indicate that definitely this is not always the case. On the other hand, insoluble catalysts can be easily recovered and thus more easily recycled than their homogeneous analogues. Moreover, a number of organic–inorganic POM hybrid catalysts have solubility properties that can be varied by changing the environmental conditions, such as the medium polarity and reaction temperature, thus coupling the advantages of homogeneous and heterogeneous systems. These compounds represent a type of self-supported heterogeneous catalyst with highly tunable structural features of acidity, porosity, charge density and surface properties. The catalytic activity is another factor that should be considered in this matter. As mentioned in Sections 2.1 and 2.2, porosity, redox and acidity of the HPC can be tuned with changing the counter cation and thus, a list of suggestions depends on the reaction type.
From this discussion, the heterogenization of HPCs emerges as a field of research still in its infancy and open to great expansion in the future, provided that inorganic, organic, polymer and material chemists are able to combine their efforts in a multidisciplinary approach.
Conclusions
The heterogenization of different kinds of HPCs via a wide variety methods was reviewed. The main processes for this goal are dispersion, encapsulation, precipitation, tethering and grafting. All of these ways have been greatly improved over the last 15 years, especially when HPCs are produced as nano-size catalysts. Significant progress is being made in several key research areas, such as by using a wide variety of supports for the heterogenizations, including silica, alumina, carbon, zeolite, MOF, etc. Also, improvement of the preparation methods provides a wide subject area for scientists to increase the ability of using such important catalysts in industrial processes, because easy recovery of the catalysts and the reuse possibility with no significant change in activity or selectivity classify these compounds as green catalysts. In this review, all the methods for the heterogenization of HPCs and related compounds have been summarized to support further improvements in this subject area and to facilitate extending these methods to other homogeneous catalysts.Acknowledgements
The authors thank the Razi University Research Council for support of this work.Notes and references
- M. T. Pope, Heteropoly and Isopoly Oxometalates, Springer-Verlag, Berlin, 1983 Search PubMed.
- J. B. Moffat, Metal-Oxygen Clusters: the Surface and Catalytic Properties of Heteropoly Oxometalates, Springer, 2002 Search PubMed.
- G. A. Tsigdinos, Heteropoly compounds of molybdenum and tungsten, Topics in current chemistry, 2006, vol. 76 Search PubMed.
- M. Misono, Catalysis of Heteropoly Compounds (Polyoxometalates), Studies in Surface Science and Catalysis, 2013, vol. 176 Search PubMed.
- J. J. Borras-Almenar, E. Coronado, A. Muller and M. Pope, Polyoxometalate Molecular Science, NATO Science Series, 2003, vol. 98 Search PubMed.
- T. Okuhara and T. Nakato, Catal. Surv. Asia, 1998, 2, 31 CrossRef CAS.
- T. Okuhara, N. Mizuno and M. Misono, Adv. Catal., 1996, 41, 113 CAS.
- T. Okuhara, N. Mizuno and M. Misono, Appl. Catal., A, 2001, 222, 63 CrossRef CAS.
- T. J. Barton, L. M. Bull, W. G. Klemperer, D. A. Loy, B. McEnaney, M. Misono, P. A. Monson, G. Pez, G. W. Scherer, J. C. Vartuli and O. M. Yaghi, Chem. Mater., 1999, 11, 2633 CrossRef CAS.
- I. V. Kozhevnikov, Catal. Rev.: Sci. Eng., 1995, 37, 311 CAS.
- E. Y. Safronova, A. K. Osipov, A. E. Baranchikov and A. B. Yaroslavtsev, Inorg. Mater., 2015, 51, 1157 CrossRef CAS.
- G. Hecquet, Catal. Lett., 1995, 32, 215 CrossRef.
- T. Okuhara, H. Watanabe, T. Nishimura, K. Inumaru and M. Misono, Chem. Mater., 2000, 12, 2230 CrossRef CAS.
- M. Misono, Chem. Commun., 2001, 1141 RSC.
- V. Z. Sasca, O. Verdes, L. Avram, A. Popa, A. Erdőhelyi and A. Oszko, Appl. Catal., A, 2013, 451, 50 CrossRef CAS.
- E. Rafiee, Z. Zolfagharifar, M. Joshaghani and S. Eavani, Appl. Catal., A, 2009, 365, 287 CrossRef.
- E. Rafiee, S. Eavani, F. Khajooei Nejad and M. Joshaghani, Tetrahedron, 2010, 66, 6858 CrossRef CAS.
- E. Rafiee, F. Khajooei Nejad and M. Joshaghani, Chin. Chem. Lett., 2011, 22, 288 CrossRef CAS.
- E. Rafiee, M. Khodayari and M. Joshaghani, Can. J. Chem., 2011, 89, 1533 CrossRef CAS.
- E. Rafiee and F. Rahimi, Monatsh. Chem., 2013, 144, 361 CrossRef CAS.
- E. Rafiee and M. Kahrizi, S. Afr. J. Chem., 2013, 66, 145 CAS.
- H. W. Park, S. Park, D. R. Park, J. H. Choi and I. K. Song, Catal. Commun., 2010, 12, 1 CrossRef CAS.
- Y. Iwase, S. Sano, L. Mahardiani, R. Abe and Y. Kamiya, J. Catal., 2014, 318, 34 CrossRef CAS.
- G. Maayan, B. Ganchegui, W. Leitner and R. Neumann, Chem. Commun., 2006, 2230 RSC.
- I. V. Kozhevnikov, Catalysts for Fine Chemicals, Catalysis by Polyoxometalates, Wiley, Chichester, 2002, vol. 2 Search PubMed.
- H. Benaissa, P. N. Davey, Y. Z. Khimyak and I. V. Kozhevnikov, J. Catal., 2008, 253, 244 CrossRef CAS.
- D. A. Friesen, D. B. Gibson and C. H. Langford, Chem. Commun., 1998, 543 RSC.
- D. A. Friesen, L. Morello, J. V. Headley and C. H. Langford, J. Photochem. Photobiol., A, 2000, 133, 213 CrossRef CAS.
- D. A. Friesen, Chem. Commun., 1998, 543 RSC.
- J. Poźniczek, A. Lubańska, D. Mucha and A. Bielański, J. Mol. Catal. A: Chem., 2006, 257, 99 CrossRef.
- X. Yu, Y. Guo, K. Li, X. Yang, L. Xu, Y. Guo and J. Hu, J. Mol. Catal. A: Chem., 2008, 290, 44 CrossRef CAS.
- E. Rafiee, S. Tangestaninejad, M. H. Habibi and V. Mirkhani, Bioorg. Med. Chem. Lett., 2004, 13, 3611 CrossRef PubMed.
- E. Rafiee, S. Tangestaninejad, M. H. Habibi and V. Mirkhani, Bull. Korean Chem. Soc., 2004, 25, 599 CrossRef CAS.
- E. Rafiee, S. Tangestaninejad, M. H. Habibi and V. Mirkhani, Synth. Commun., 2004, 34, 3673 CrossRef CAS.
- E. Rafiee, S. Tangestaninejad, M. H. Habibi, I. Mohammadpoor-Baltork and V. Mirkhani, Russ. J. Org. Chem., 2005, 41, 403 CrossRef.
- E. Rafiee, S. Tangestaninejad, M. H. Habibi and V. Mirkhani, Bull. Korean Chem. Soc., 2005, 26, 1585 CrossRef CAS.
- E. Rafiee and A. Azad, Synth. Commun., 2007, 37, 1127 CrossRef.
- E. Rafiee, A. Azad and M. Joshaghani, Lett. Org. Chem., 2007, 4, 60 CrossRef CAS.
- E. Rafiee and A. Azad, Bioorg. Med. Chem. Lett., 2007, 17, 2756 CrossRef CAS PubMed.
- E. Rafiee, Z. Zolfagharifar, M. Joshaghani and S. Eavani, S. Afr. J. Chem., 2012, 65, 138 CAS.
- E. Rafiee, M. Kahrizi and M. Joshaghani, Chin. Chem. Lett., 2012, 23, 1363 CrossRef CAS.
- T. Kasza and A. Bielański, Catal. Lett., 2009, 128, 307 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy, K. V. Purnima, S. Jhansi, K. Nagaiah and N. Lingaiah, Catal. Commun., 2008, 9, 2361 CrossRef CAS.
- K. M. Reddy, N. S. Babu, I. Suryanarayana, P. S. S. Prasad and N. Lingaiah, Tetrahedron Lett., 2006, 47, 7563 CrossRef CAS.
- S. Borghèse, B. Louis, A. Blanc and P. Pale, Catal. Sci. Technol., 2011, 1, 981 Search PubMed.
- M. A. Parent and J. B. Moffat, Catal. Lett., 1997, 48, 135 CrossRef CAS.
- N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed.
- S. Yoshida, H. Niiyama and E. Echigoya, J. Chem. Phys., 1982, 86, 3150 CrossRef CAS.
- M. Hanaya, T. Takahashi and T. Baba, J. Mol. Struct., 2002, 602–603, 367 CrossRef CAS.
- T. Baba and Y. Ono, J. Phys. Chem., 1996, 100, 9064 CrossRef CAS.
- D. Mucha, L. Matachowski, T. Machej, J. Gurgul and R. P. Socha, Solid State Sci., 2011, 13, 1276 CrossRef CAS.
- J. T. Rhule, W. A. Neiwert, K. I. Hardcastle, B. T. Do and C. L. Hill, J. Am. Chem. Soc., 2011, 123, 12101 CrossRef.
- A. W. Stobbe-Kreemers, G. van der Lans, M. Makkee and J. J. F. Scholten, J. Catal., 1995, 154, 187 CrossRef.
- I. V. Kozhevnikov and K. I. Matveev, Russ. Chem. Rev., 1978, 47, 649 CrossRef.
- I. V. Kozhevnikov, Russ. Chem. Rev., 1983, 52, 138 CrossRef.
- H. A. Burton and I. V. Kozhevnikov, J. Mol. Catal. A: Chem., 2002, 185, 285 CrossRef CAS.
- D. Baratsa and R. Neumann, Adv. Synth. Catal., 2010, 352, 293 CrossRef.
- K. Nowińska and D. Dudko, Appl. Catal., A, 1997, 159, 75 CrossRef.
- T. Yamada, A. Sakakura, S. Sakaguchi, Y. Obora and Y. Ishii, New J. Chem., 2008, 32, 738 RSC.
- X. Li, Y. Ding, G. Jiao, Z. Pan, D. Jiang and L. Lin, Chin. J. Catal., 2005, 26, 265 Search PubMed.
- R. A. De Oliveira, E. V. Gusevskaya and F. Carazza, J. Braz. Chem. Soc., 2002, 13, 110 CrossRef CAS.
- V. Kogan, Z. Aizenshtat, R. Popovitz-Biro and R. Neumann, Org. Lett., 2002, 4, 3529 CrossRef CAS PubMed.
- T. Yokota, S. Sakaguchi and Y. Ishii, Adv. Synth. Catal., 2002, 344, 849 CrossRef CAS.
- A. Srivani, P. S. S. Prasad and N. Lingaiah, Catal. Lett., 2012, 142, 389 CrossRef CAS.
- H. Zhang, X. Zhang, Y. Ding, L. Yan, T. Ren and J. Suo, New J. Chem., 2002, 26, 376 RSC.
- D. S. Mansilla, M. R. Torviso, E. N. Alesso, P. G. Vázquez and C. V. Cáceres, Appl. Catal., A, 2010, 375, 196 CrossRef CAS.
- D. Rath, S. Rana and K. M. Parida, Ind. Eng. Chem. Res., 2010, 49, 8942 CrossRef CAS.
- K. V. Purnima, D. Sreenu, N. Bhasker, K. Nagaiah, N. Lingaiah, B. V. S. Reddy and J. S. Yadav, Chin. J. Chem., 2013, 31, 534 CrossRef CAS.
- A. Aouissi, L. A. Allaoui and D. Aldhayan, Asian J. Chem., 2006, 18, 3009 CAS.
- A. Aouissi, D. Al-Dhayan, Z. A. Al-Othman and A. Lokbaichi, Asian J. Chem., 2012, 24, 3899 CAS.
- F. Chen, T. Dong, Y. Chi, Y. Xu and C. Hu, Catal. Lett., 2010, 139, 38 CrossRef CAS.
- A. Popa, V. Sasca, D. Bajuk-Bogdanović and I. Holclajtner-Antunović, J. Porous Mater., 2016, 23, 211 CrossRef CAS.
- P. A. Nikul'shin, A. V. Mozhaev, A. A. Pimerzin, N. N. Tomina, V. V. Konovalov and V. M. Kogan, Kinet. Catal., 2011, 52(2011), 862 CrossRef.
- L. Wang, L. Yue, F. Shi, J. Guo, J. Yang, J. Lian, X. Luo and Y. Guo, Water Sci. Technol., 2015, 71, 848 CrossRef CAS PubMed.
- A. Forment-Aliaga, E. Coronado, M. Feliz, A. Gaita-Ariño, R. Llusar and F. M. Romero, Inorg. Chem., 2003, 42, 8019 CrossRef CAS PubMed.
- K. Nowińska, React. Kinet. Catal. Lett., 1997, 61, 187 CrossRef.
- H. K. Pamin, J. Połtowicz, M. Prończuk, S. Basag, J. Maciejewska, J. Krýsciak-Czerwenka and R. Tokarz-Sobieraj, Catal. Today, 2015, 257, 80 CrossRef.
- A. Aouissi, S. S. Al-Deyab, A. Al-Owais and A. Al-Amro, Int. J. Mol. Sci., 2010, 11, 2770 CrossRef CAS PubMed.
- U. Filek, E. Bielańska, R. P. Socha and A. Bielański, Catal. Today, 2011, 169, 150 CrossRef CAS.
- K. Shimizu, K. Niimi and A. Satsuma, Appl. Catal., A, 2008, 349, 1 CrossRef CAS.
- K. Shimizu, K. Niimi and A. Satsuma, Catal. Commun., 2008, 9, 980 CrossRef CAS.
- K. M. Reddy, N. S. Babu, P. S. S. Prasad and N. Lingaiah, Catal. Commun., 2008, 9, 2525 CrossRef CAS.
- Z. Zhu and W. Yang, J. Phys. Chem. C, 2009, 113, 17025 CAS.
- C. R. Kumar, P. S. S. Prasad and N. Lingaiah, J. Mol. Catal. A: Chem., 2011, 350, 83 CrossRef.
- C. R. Kumar, K. Jagadeeswaraiah, P. S. S. Prasad and N. Lingaiah, ChemCatChem, 2012, 4, 1360 CrossRef CAS.
- C. Xiaohua, R. Jie, X. Changlong, Z. Kanghua, Z. Changchao and L. Jian, Chin. J. Chem. Eng., 2013, 21, 500 CrossRef.
- J. S. Santos, J. A. Dias, S. C. L. Dias, F. A. C. Garcia, J. L. Macedo, F. S. G. Sousa and L. S. Almeida, Appl. Catal., A, 2011, 394, 138 CrossRef CAS.
- J. S. Santos, J. A. Dias, S. C. L. Dias, J. L. de Macedo, F. A. C. Garcia, L. S. Almeida and E. N. C. B. de Carvalho, Appl. Catal., A, 2012, 443–444, 33 CrossRef CAS.
- X. Chen, H. Wang, J. Xu, M. Huo, Z. Jiang and X. Wang, Catal. Today, 2014, 234, 264 CrossRef CAS.
- S. Park, S. H. Lee, S. H. Song, D. R. Park, S. H. Baeck, T. J. Kim, Y. M. Chung, S. H. Oh and I. K. Song, Catal. Commun., 2009, 10, 391 CrossRef CAS.
- F. X. Liu-Cai, C. Pham, F. Bey and G. Hervé, React. Kinet. Catal. Lett., 2002, 75, 305 CrossRef CAS.
- Q. Huynh, Y. Schuurman, P. Delichere, S. Loridant and J. M. M. Millet, J. Catal., 2009, 261, 166 CrossRef CAS.
- S. Loridant, Q. Huynh and J. M. M. Millet, Catal. Today, 2010, 155, 214 CrossRef CAS.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS PubMed.
- R. Neumann and M. Dahan, Nature, 1997, 388, 353 CrossRef CAS.
- R. A. Sheldon, I. W. C. E. Arends and A. Dijksman, Catal. Today, 2000, 57, 157 CrossRef CAS.
- L. D. Dingwall, C. M. Corcoran, A. F. Lee, L. Olivi, J. M. Lynam and K. Wilson, Catal. Commun., 2008, 10, 53 CrossRef CAS.
- Y. Leng, J. Liu, P. Jiang and J. Wang, Chem. Eng. J., 2014, 239, 1 CrossRef CAS.
- L. R. Cao, K. P. O'Halloran, H. Ma and L. Wu, Electrochim. Acta, 2008, 54, 484 CrossRef.
- H. Tan, W. Chen, Y. G. Li, D. Liu, L. Chen and E. Wang, J. Solid State Chem., 2009, 182, 465 CrossRef CAS.
- F. Li, L. Xu, Y. Wei, Y. Qiu, Z. Wang and E. Wang, J. Coord. Chem., 2005, 58, 1751 CrossRef CAS.
- A. Sartorel, M. Carraro, G. Scorrano, R. De Zorzi, S. Geremia, N. D. McDaniel, S. Bernhard and M. Bonchio, J. Am. Chem. Soc., 2008, 130, 5006 CrossRef CAS PubMed.
- Z. Huo, J. Zhao, Z. Bu, P. Ma, Q. Liu, J. Niu and J. Wang, ChemCatChem, 2014, 6, 3096 CrossRef CAS.
- J. Niu, S. Zhang, H. Chen, J. Zhao, P. Ma and J. Wang, Cryst. Growth Des., 2011, 11, 3769 CAS.
- S. M. Sadeghzadeh, Res. Chem. Intermed., 2016, 42, 2317 CrossRef CAS.
- P. G. Rickert, M. R. Antonio, M. A. Firestone, K. A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, J. Phys. Chem. B, 2007, 111, 4685 CrossRef CAS PubMed.
- P. G. Rickert, M. R. Antonio, M. A. Firestone, K. A. Kubatko, T. Szreder, J. F. Wishart and M. L. Dietz, Dalton Trans., 2007, 529 RSC.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- H. Ohno, Electrochemical aspects of ionic liquids, ed. H. Ohno, Wiley, New York, 2005 Search PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed.
- D. Chen, A. Sahasrabudhe, P. Wang, A. Dasgupta, R. Yuan and S. Roy, Dalton Trans., 2013, 42, 10587 RSC.
- L. Liu, C. Chen, X. Hu, T. Mohamood, W. Ma, J. Lin and J. Zhao, New J. Chem., 2008, 32, 283 RSC.
- A. Corma, S. Iborra, F. X. L. Xamena, R. Montón, J. J. Calvino and C. Prestipino, J. Phys. Chem. C, 2010, 114, 8828 CAS.
- M. Rostami, A. R. Khosropour, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moghadam and Sh. Tangestaninejad, C. R. Chimi., 2011, 14, 869 CrossRef CAS.
- H. Li, Y. Qiao, L. Hua, Z. Hou, B. Feng, Z. Pan, Y. Hu, X. Wang, X. Zhao and Y. Yu, ChemCatChem, 2010, 2, 1165 CrossRef CAS.
- T. Rajkumar and G. R. Rao, Chem. Sci., 2008, 120, 587 CrossRef CAS.
- M. Rostami, A. Khosropour, V. Mirkhani, M. Moghadam, Sh. Tangestaninejad and I. Mohammadpoor-Baltork, Appl. Catal., A, 2011, 397, 27 CrossRef CAS.
- J. Sun, H. Liu, K. Yao, J. Ma and J. Wu, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2005, 35, 527 CrossRef CAS.
- D. J. Qian, H. X. Huang, W. Huang, T. Wakayama, C. Nakamura and J. Miyake, Colloids Surf., A, 2004, 248, 85 CrossRef CAS.
- K. Shimizu, S. Kontani, S. Yamada, G. Takahashi, T. Nishiyama and A. Satsuma, Appl. Catal., A, 2010, 380, 33 CrossRef CAS.
- D. Chen, Q. Zhang, G. Wang, H. Zhang and J. Li, Electrochem. Commun., 2007, 9, 2755 CrossRef CAS.
- Y. Leng, J. Wang, D. Zhu, M. Zhang, P. Zhao, Z. Long and J. Huang, Green Chem., 2011, 13, 1636 RSC.
- G. E. H. Qing, L. Yan, Z. F. Min, P. J. Rui, Z. C. Jiang and W. Jun, Sci. China, Ser. B: Chem., 2009, 52, 1264 Search PubMed.
- W. Huang, W. Zhu, H. Li, H. Shi, G. Zhu, H. Liu and G. Chen, Ind. Eng. Chem. Res., 2010, 49, 8998 CrossRef CAS.
- H. Li, Y. Qiao, L. Hua, Z. Hou, B. Feng, Z. Pan, Y. Hu, X. Wang, X. Zhao and Y. Yu, ChemCatChem, 2010, 2, 1165 CrossRef CAS.
- Y. Leng, J. Wang, D. Zhu, Y. Wu and P. Zhao, J. Mol. Catal. A: Chem., 2009, 313, 1 CrossRef CAS.
- E. Rafiee and F. Mirnezami, J. Mol. Liq., 2014, 199, 156 CrossRef CAS.
- Z. Changjiang, G. Hanqing, L. Yan, W. Jun and L. Chain, Chin. J. Catal., 2010, 31, 623 CrossRef.
- W. Zhu, W. Huang, H. Li, M. Zhang, W. Jiang, G. Chen and C. Han, Fuel Process. Technol., 2011, 92, 1842 CrossRef CAS.
- W. Zhu, G. Zhu, H. Li, Y. Chao, M. Zhang, D. Du, Q. Wang and Z. Zhao, Fuel Process. Technol., 2013, 106, 70 CrossRef CAS.
- X. Lia, R. Cao and Q. Lin, Catal. Commun., 2015, 69, 5 CrossRef.
- Y. Dai, B. D. Li, H. D. Quan and C. X. Lu, Chin. Chem. Lett., 2010, 21, 678 CrossRef CAS.
- N. Sun, X. Jiang, M. L. Maxim, A. Metlen and R. D. Rogers, ChemSusChem, 2011, 4, 65 CrossRef CAS PubMed.
- H. Lü, W. Ren, H. Wang, Y. Wang, W. Chen and Z. Suo, Appl. Catal., A, 2013, 453, 376 CrossRef.
- H. Lü, C. Deng, W. Z. Ren and X. Yang, Fuel Process. Technol., 2014, 119, 87 CrossRef.
- Y. Ding, W. Zhu, H. Li, W. Jiang, M. Zhang, Y. Duan and Y. Chang, Green Chem., 2011, 13, 1210 RSC.
- J. Qiu, G. Wang, D. Zeng, Y. Tang, M. Wang and Y. Li, Fuel Process. Technol., 2009, 90, 1538 CrossRef CAS.
- C. Jiang, J. Wang, S. Wang, H. yu Guan, X. Wang and M. Huo, Appl. Catal., B, 2011, 106, 343 CrossRef CAS.
- Y. Lu, Z. Sun and M. Huo, RSC Adv., 2015, 5, 30869 RSC.
- J. Langanke, L. Greiner and W. Leitner, Green Chem., 2013, 15, 1173 RSC.
- E. Rafiee and S. Eavani, J. Mol. Catal. A: Chem., 2013, 380, 18 CrossRef CAS.
- X. Chen, B. Souvanhthong, H. Wang, H. Zheng, X. Wang and M. Huo, Appl. Catal., B, 2013, 138–139, 161 CrossRef CAS.
- E. Rafiee and S. Eavani, Catal. Commun., 2012, 25, 64 CrossRef CAS.
- K. Li, L. Chen, H. Wang, W. Lin and Z. Yan, Appl. Catal., A, 2011, 392, 233 CrossRef CAS.
- E. Rafiee and S. Eavani, J. Mol. Liq., 2014, 199, 96 CrossRef CAS.
- J. Chen, L. Hua, W. Zhu, R. Zhang, L. Guo, C. Chen, H. Gan, B. Song and Z. Hou, Catal. Commun., 2014, 47, 18 CrossRef CAS.
- F. Mohammadi Zonoz, A. Jamshidi and S. Tavakoli, Solid State Sci., 2013, 17, 83 CrossRef CAS.
- H. Ma, J. Peng, Z. Han, X. Yu and B. Dong, J. Solid State Chem., 2005, 178, 3735 CrossRef CAS.
- Y. Leng, P. Jiang and J. Wang, Catal. Commun., 2012, 25, 41 CrossRef CAS.
- P. Zhao, M. Zhang, Y. Wu and J. Wang, Ind. Eng. Chem. Res., 2012, 51, 6641 CrossRef CAS.
- J. Li, Y. Zhou, D. Mao, G. Chen, X. Wang, X. Yang, M. Wang, L. Peng and J. Wang, Chem. Eng. J., 2014, 254, 54 CrossRef CAS.
- A. B. Bourlinos, K. Raman, R. Herrera, Q. Zhang, L. A. Archer and E. P. Giannelis, J. Am. Chem. Soc., 2004, 126, 15358 CrossRef CAS PubMed.
- H. Li, Z. Hou, Y. Qiao, B. Feng, Y. Hu, X. Wang and X. Zhao, Catal. Commun., 2010, 11, 470 CrossRef CAS.
- M. Ammamn and J. Fransaer, J. Solid State Chem., 2011, 184, 818 CrossRef.
- H. Zhao, L. Zeng, Y. Li, C. Liu, B. Hou, D. Wu, N. Feng, A. Zheng, X. Xie, S. Su and N. Yu, Microporous Mesoporous Mater., 2013, 172, 67 CrossRef CAS.
- B. Zhen, H. Li, Q. Jiao, Y. Li, Q. Wu and Y. Zhang, Ind. Eng. Chem. Res., 2012, 51, 10374 CrossRef CAS.
- M. Bagheri, M. Masteri-Farahani and M. Ghorbani, J. Magn. Magn. Mater., 2013, 327, 58 CrossRef CAS.
- J. Zhu, P. Wang and M. Lu, RSC Adv., 2012, 2, 8265 RSC.
- X. Li, R. Cao and Q. Lin, Catal. Commun., 2015, 63, 79 CrossRef CAS.
- X. Han, K. Chen, H. Du, X. J. Tang, C. T. Hung, K. Chen Lin and S. B. Liu, J. Taiwan Inst. Chem. Eng., 2016, 58, 203 CrossRef CAS.
- F.-L. Yu, C.-Y. Liu, P.-H. Xie, B. Yuan, C.-X. Xie and S.-T. Yu, RSC Adv., 2015, 5, 85540 RSC.
- E. F. Kozhevnikova, E. Rafiee and I. V. Kozhevnikov, Appl. Catal., A, 2004, 260, 25 CrossRef CAS.
- J. Kaur, K. Griffin, B. Harrison and I. V. Kozhevnikov, J. Catal., 2002, 208, 448 CrossRef CAS.
- E. Rafiee, M. Joshaghani, S. Eavani and S. Rashidzadeh, Green Chem., 2008, 10, 982 RSC.
- E. Rafiee, F. Shahbazi, M. Joshaghani and F. Tork, J. Mol. Catal. A: Chem., 2005, 242, 129 CrossRef CAS.
- E. Rafiee and F. Shahbazi, J. Mol. Catal. A: Chem., 2006, 250, 57 CrossRef CAS.
- R. Tayebee and E. Rafiee, Bull. Chem. Soc. Ethiop., 2006, 20, 329 CrossRef CAS.
- E. Rafiee, S. Rashidzadeh and A. Azad, J. Mol. Catal. A: Chem., 2006, 260, 49 CrossRef.
- E. Rafiee and F. Rahimi, J. Chil. Chem. Soc., 2013, 58, 1926 CrossRef CAS.
- E. Rafiee, A. Fakhri and M. Joshaghani, J. Heterocycl. Chem., 2013, 50, 1121 CAS.
- E. Rafiee, F. Khajooei Nejad and M. Joshaghani, S. Afr. J. Chem., 2011, 64, 95 CAS.
- E. Rafiee, Z. Zolfagharifar, M. Joshaghani and S. Eavani, Synth. Commun., 2011, 41, 459 CrossRef CAS.
- E. Rafiee, H. Mahdavi and M. Joshaghani, S. Afr. J. Chem., 2010, 63, 135 Search PubMed.
- E. Rafiee, M. Joshaghan, F. Tork, A. Fakhri and S. Eavani, J. Mol. Catal. A: Chem., 2008, 283, 1 CrossRef CAS.
- E. Rafiee, F. Paknezhad, Sh. Shahebrahimi, M. Joshaghani, S. Eavani and S. Rashidzadeh, J. Mol. Catal. A: Chem., 2008, 282, 92 CrossRef CAS.
- E. Rafiee, S. Eavani, S. Rashidzadeh and M. Joshaghani, Inorg. Chim. Acta, 2009, 362, 3555 CrossRef CAS.
- E. Rafiee, H. Mahdavi and M. Joshaghani, Mol. Diversity, 2011, 15, 125 CrossRef CAS PubMed.
- E. Rafiee, Sh. Shahebrahimi, M. Feyzi and M. Shaterzadeh, Int. Nano Lett., 2012, 2, 29 CrossRef.
- E. Rafiee and Sh. Shahebrahimi, Chin. J. Catal., 2012, 33, 1326 CrossRef CAS.
- E. Rafiee, M. Khodayari, Sh. Shahebrahimi and M. Joshaghani, J. Mol. Catal. A: Chem., 2011, 351, 204 CrossRef CAS.
- E. Rafiee, M. Khodayari, M. Kahrizi and R. Tayebee, J. Mol. Catal. A: Chem., 2012, 358, 121 CrossRef CAS.
- E. Rafiee and M. Kahrizi, Res. Chem. Intermed., 2015, 41, 2833 CrossRef CAS.
- E. Rafiee and F. Jalilian, Lett. Org. Chem., 2014, 11, 203 CrossRef CAS.
- E. Rafiee and M. Kahrizi, Res. Chem. Intermed., 2016, 42, 1125 CrossRef CAS.
- G. D. Yadav and H. G. Manyar, Microporous Mesoporous Mater., 2003, 63, 85 CrossRef CAS.
- T. Blasco, A. Corma, A. Martínez and P. Martínez-Escolano, J. Catal., 1998, 177, 306 CrossRef CAS.
- A. Nemati Kharat, P. Pendleton, A. Badalyan, M. Abedini and M. Mohammadpour Amini, J. Mol. Catal. A: Chem., 2001, 175, 277 CrossRef.
- Á. Kukovecz, Zs. Balogi, Z. Kónya, M. Toba, P. Lentz, S.-I. Niwa, F. Mizukami, Á. Molnár, J. B. Nagy and I. Kiricsi, Appl. Catal., A, 2002, 228, 83 CrossRef.
- J. Yuan, P. Yue and L. Wang, Powder Technol., 2010, 202, 190 CrossRef CAS.
- Y. Chen, Y. Cao, G. P. Zheng, B. B. Dong and X. C. Zheng, Adv. Powder Technol., 2014, 25, 1351 CrossRef CAS.
- A. Popa, V. Sasca, D. Bajuk-Bogdanović and I. Holclajtner-Antunović, J. Porous Mater., 2016, 23, 211 CrossRef CAS.
- M. T. Pope, Compr. Coord. Chem. II, 2004, 4, 635 CAS.
- C. L. Hill, Compr. Coord. Chem. II, 2004, 4, 679 CAS.
- R. R. Ozer and J. L. Ferry, Environ. Sci. Technol., 2001, 35, 3242 CrossRef CAS PubMed.
- C. Chen, P. Lei, H. Ji, W. Ma, J. Zhao, H. Hidaka and N. Serpone, Environ. Sci. Technol., 2004, 38, 329 CrossRef CAS PubMed.
- W. B. Kim, T. Voitl, G. J. Rodriguez-Rivera and J. A. Dumesic, Science, 2004, 305, 1280 CrossRef CAS PubMed.
- C. Gu and C. Shannon, J. Mol. Catal. A: Chem., 2007, 262, 185 CrossRef CAS.
- A. Pearson, H. Zheng, K. Kalantar-zadeh, S. K. Bhargava and V. Bansal, Langmuir, 2012, 28, 14470 CrossRef CAS PubMed.
- Y. Le, Y. Shuai, S. Liyi, Z. Yin and F. Jianhui, Microporous Mesoporous Mater., 2010, 134, 108 CrossRef.
- C. R. Kumar, P. S. S. Prasad and N. Lingaiah, J. Mol. Catal. A: Chem., 2011, 350, 83 CrossRef.
- R. M. Ladera, M. Ojeda, J. L. G. Fierro and S. Rojas, Catal. Sci. Technol., 2015, 5, 484 CAS.
- G. Marcì, E. I. G. López and L. Palmisano, Eur. J. Inorg. Chem., 2014, 21 CrossRef.
- E. Rafiee, H. Mahdavi, S. Eavani, M. Joshaghani and F. Shiri, Appl. Catal., A, 2009, 352, 202 CrossRef CAS.
- E. Rafiee, S. Eavani, S. Rashidzadeh and M. Joshaghani, Heterocycles, 2008, 75, 2225 CrossRef CAS.
- M. E. Pérez, D. M. Ruiz, J. C. Autino, M. N. Blanco, L. R. Pizzio and G. P. Romanelli, J. Porous Mater., 2013, 20, 1433 CrossRef.
- N. Toutounchian, A. Ahmadpour, M. M. Heravi, F. F. Bamoharram, A. Ayati and F. Deymeh, Res. Chem. Intermed., 2016, 42, 3283 CrossRef CAS.
- R. M. Ladera, M. Ojeda, J. L. G. Fierro and S. Rojas, Catal. Sci. Technol., 2015, 5, 484 CAS.
- I. V. Kozhevnikov, A. Sinnema, R. J. J. Jansen and H. van Bekkum, Catal. Lett., 1994, 27, 187 CrossRef CAS.
- Y. Izumi and K. Urabe, Chem. Lett., 1981, 663 CrossRef CAS.
- M. A. Schwegler, H. van Bekkum and N. A. Munck, Appl. Catal., 1991, 74, 191 CAS.
- M. A. Schwegler, P. Vinke, M. van der Eijk and H. van Bekkum, Appl. Catal., A, 1992, 80, 41 CrossRef CAS.
- J. A. Monge, G. Trautwein, S. Parres-Esclapez and J. A. M. Agulló, Microporous Mesoporous Mater., 2008, 115, 440 CrossRef.
- X. L. Long, Z. H. Wang, S. Q. Wu, S. M. Wu, H. F. Lv and W. K. Yuan, J. Ind. Eng. Chem., 2014, 20, 100 CrossRef CAS.
- R. J. J. Jansen, H. M. van Veldhuizen and H. van Bekkum, J. Mol. Catal. A: Chem., 1996, 107, 241 CrossRef CAS.
- Z. Obalı and T. Dógu, Chem. Eng. J., 2008, 138, 548 CrossRef.
- E. Rafiee, S. Eavani, E. Babaee, F. Toodehroosta and K. Daneshpazhuh, Z. Naturforsch., 2008, 63b, 178 Search PubMed.
- E. Rafiee, S. Eavani and M. Joshaghani, J. Carbohydr. Chem., 2010, 29, 20 CrossRef CAS.
- E. Rafiee, M. Joshaghani and P. Ghaderi-Shekhi Abadi, Arabian J. Chem. DOI:10.1016/j.arabjc.2015.09.004.
- X. H. Wang, F. Li, J. F. Liu and M. T. Pope, Chin. Chem. Lett., 2004, 15, 714 CAS.
- E. Rafiee and M. Khodayari, Res. Chem. Intermed., 2016, 42, 3523 CrossRef CAS.
- V. A. Likholobov, V. B. Fenelonov, O. G. Okkel, O. V. Goncharova, L. B. Aavdeeva, V. I. Zaikovskii, G. G. Kuvshinov, V. A. Semikolenov, V. K. Duplyakin, O. N. Baklanova and G. V. Plaksin, React. Kinet. Catal. Lett., 1995, 54, 381 CrossRef CAS.
- V. B. Fenelonov, A. Y. Derevyankin, L. G. Okkel, L. B. Avdeeva, V. I. Zaikovskii, E. M. Moroz, A. N. Salanov, N. A. Rudina, V. A. Likholobov and Sh. K. Shaikhutdinov, Carbon, 1997, 33, 1140 Search PubMed.
- M. N. Timofeeva, M. M. Matrosova, G. N. I'Iinich, T. V. Reshetenko, L. B. Avdeeva, R. I. Kvon, A. L. Chuvilin, A. A. Budneva, E. A. Pauk-shtis and V. A. Likholobov, Kinetika i Kataliz., 2003, 44, 1 Search PubMed.
- M. N. Timofeeva, M. M. Matrosova, T. V. Reshetenko, L. B. Avdeeva, E. A. Paukshtis, A. A. Budneva, A. L. Chuvilin and V. A. Likholobov, Russ. Chem. Bull., 2002, 51, 243 CrossRef CAS.
- Y. A. Ryndin, O. S. Alekseev, P. A. Simonov and V. A. Likholobov, J. Mol. Catal., 1989, 55, 109 CrossRef CAS.
- M. N. Timofeeva, M. M. Matrosova, T. V. Reshetenko, L. B. Avdeeva, A. A. Budneva, A. B. Ayupov, E. A. Paukshtis, A. L. Chuvilin, A. V. Volodin and V. A. Likholobov, J. Mol. Catal. A: Chem., 2004, 211, 131 CrossRef CAS.
- G. D. Yadav, Catal. Surv. Asia, 2005, 9, 117 CrossRef CAS.
- G. D. Yadav and V. V. Bokade, Appl. Catal., A, 1996, 147, 299 CrossRef CAS.
- G. D. Yadav and A. A. Pujari, Green Chem., 1999, 1, 69 RSC.
- G. D. Yadav and P. K. Goel, Green Chem., 2000, 2, 71 RSC.
- G. D. Yadav, P. K. Goel and A. V. Joshi, Green Chem., 2001, 3, 92 RSC.
- G. D. Yadav and N. S. Doshi, Org. Process Res. Dev., 2002, 6, 263 CrossRef CAS.
- H. Gurav and V. V. Bokade, J. Nat. Gas Chem., 2010, 19, 161 CrossRef CAS.
- V. V. Bokade and G. D. Yadav, Appl. Clay Sci., 2011, 53, 263 CrossRef CAS.
- E. Rafiee, S. Rashidzadeh, S. Eavani and M. Joshaghani, Bull. Chem. Soc. Ethiop., 2010, 24, 209 CrossRef CAS.
- V. V. Bokade and G. D. Yadav, J. Mol. Catal. A: Chem., 2008, 285, 155 CrossRef CAS , and references cited in.
- M. Nehate and V. V. Bokade, Appl. Clay Sci., 2009, 44, 255 CrossRef CAS.
- K. Pamin, A. Kubacka, Z. Olejniczak, J. Haber and B. Sulikowski, Appl. Catal., A, 2000, 194–195, 137 CrossRef CAS.
- S. K. Bhorodwaj and D. K. Dutta, Appl. Catal., A, 2010, 378, 221 CrossRef CAS.
- F. Zhang, C. Yuan, J. Wang, Y. Kong, H. Zhu and C. Wang, J. Mol. Catal. A: Chem., 2006, 247, 130 CrossRef CAS.
- H. Mao, K. Zhu, X. Lu, G. Zhang, C. Yao, Y. Kong and J. Liu, J. Colloid Interface Sci., 2015, 446, 141 CrossRef CAS PubMed.
- R. Tayebee and M. Jarrahi, RSC Adv., 2015, 5, 21206 RSC.
- E. Lopez-Salinas, J. G. Hermandez-Cortez, M. A. Cortes-Jacome, J. Navarrete, M. E. Llanos, A. Vazquez, H. Armendariz and T. Lopez, Appl. Catal., A, 1998, 175, 43 CrossRef CAS.
- E. Lopez-Salinas, J. G. Hermandez-Cortez, I. Schifter, E. Torres-Garcia, J. Navarrete, A. Gutierrez-Carrillo, T. Lopez, P. P. Lottici and D. Bersani, Appl. Catal., A, 2000, 193, 215 CrossRef CAS.
- S. Patel, N. Purohit and A. Patel, J. Mol. Catal. A: Chem., 2003, 192, 195 CrossRef CAS.
- B. M. Devassy, F. Lefebvre and S. B. Halligudi, J. Catal., 2005, 231, 1 CrossRef CAS.
- S. Mallik, K. M. Parida and S. S. Dash, J. Mol. Catal. A: Chem., 2007, 261, 172 CrossRef CAS.
- P. Sharma and A. Patel, Bull. Mater. Sci., 2006, 29, 439 CrossRef CAS.
- G. Sunita, B. M. Devassy, A. Vinu, D. P. Sawant, V. V. Balasubramanian and S. B. Halligudi, Catal. Commun., 2008, 9, 696 CrossRef CAS.
- E. Skliri, I. N. Lykakis and G. S. Armatas, RSC Adv., 2014, 4, 8402 RSC.
- B. M. Devassy and S. B. Halligudi, J. Catal., 2005, 236, 313 CrossRef CAS.
- B. M. Devassy, F. Lefebvre and S. B. Halligudi, J. Catal., 2005, 231, 1 CrossRef CAS.
- B.-Q. Xu, J.-M. Wei, Y.-T. Yu, J.-L. Li and Q.-M. Zhu, Top. Catal., 2003, 22, 77 CrossRef CAS.
- B.-Q. Xu, J.-M. Wei, Y.-T. Yu, Y. Li, J.-L. Li and Q.-M. Zhu, J. Phys. Chem. B, 2003, 107, 5203 CrossRef CAS.
- S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, Appl. Catal., A, 2009, 353, 213 CrossRef CAS.
- B. Katryniok, S. Paul, M. Capron, C. Lancelot, V. Belliere-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 1922 RSC.
- B. Katryniok, S. Paul, V. Belliere-Baca, P. Rey and F. Dumeignil, Green Chem., 2010, 12, 2079 RSC.
- S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, Appl. Catal., A, 2009, 353, 213 CrossRef CAS.
- S.-H. Chai, H.-P. Wang, Y. Liang and B.-Q. Xu, Green Chem., 2008, 10, 1087 RSC.
- C. F. Oliveira, L. M. Dezaneti, F. A. C. Garcia, J. L. de Macedo, J. A. Dias, S. C. L. Dias and K. S. P. Alvim, Appl. Catal., A, 2010, 372, 153 CrossRef CAS.
- S. Zhu, Y. Zhu, X. Gao, T. Mo, Y. Zhu and Y. Li, Bioresour. Technol., 2013, 130, 45 CrossRef CAS PubMed.
- G. S. Armatas, G. Bilis and M. Louloudi, J. Mater. Chem., 2011, 21, 2997 RSC.
- G. V. Shanbhag, B. M. Devassy and S. B. Halligudi, J. Mol. Catal. A: Chem., 2004, 218, 67 CrossRef CAS.
- M. H. Haider, N. F. Dummer, D. Zhang, P. Miedziak, T. E. Davies, S. H. Taylor, D. J. Willock, D. W. Knight, D. Chadwick and G. J. Hutchings, J. Catal., 2012, 286, 206 CrossRef CAS.
- L. Liu, B. Wang, Y. Du, Z. Zhong and A. Borgna, Appl. Catal., B, 2015, 174–175, 1 CAS.
- L. Liu, B. Wang, Y. Du and A. Borgna, Appl. Catal., A, 2015, 489, 32 CrossRef CAS.
- E. Rafiee, S. Rashidzadeh, M. Joshaghani, H. Chalabeh and K. Afza, Synth. Commun., 2008, 38, 2741 CrossRef CAS.
- E. Caliman, J. A. Dias, S. C. L. Dias and A. G. S. Prado, Catal. Today, 2005, 107–108, 816 CrossRef CAS.
- S.-H. Hao, X.-Y. Zhang, D.-Q. Dong and Z.-L. Wang, Chin. Chem. Lett., 2015, 26, 599 CrossRef CAS.
- P. Villabrillea, G. Romanelli, N. Quaranta and P. Vázquez, Appl. Catal., B, 2010, 96, 379 CrossRef.
- A. Romero-Galarza, A. Gutiérrez-Alejandre and J. Ramírez, J. Catal., 2011, 280, 230 CrossRef CAS.
- B. Hari Babu, G. Parameswaram, A. Sri Hari Kumar, P. S. S. Prasad and N. Lingaiah, Appl. Catal., A, 2012, 445–446, 339 CrossRef.
- J. L. García-Gutiérrez, G. C. Laredo, P. García-Gutiérrez and F. Jiménez-Cruz, Fuel, 2014, 138, 118 CrossRef.
- M. Faraji, Y. Yamini and M. Rezaee, J. Iran. Chem. Soc., 2010, 7, 1 CrossRef CAS (and references cited therein).
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed (and references cited therein).
- S. Shyles, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef PubMed.
- M. B. Gawande, A. K. Rathi, P. S. Branco and R. S. Varma, Appl. Sci., 2013, 3, 656 CrossRef CAS (and references cited therein).
- R. B. Nasir Baig and R. S. Varma, Chem. Commun., 2013, 49, 752 RSC (and references cited therein).
- Y. Qiao, H. Li, L. Hua, L. Orzechowski, K. Yan, B. Feng, Z. Pan, N. Theyssen, W. Leitner and Z. Hou, ChemPlusChem, 2012, 77, 1128 CrossRef CAS.
- Y. Zhu, L. P. Stubbs, F. Ho, R. Liu, C. P. Ship, J. A. Maguire and N. S. Hosmane, ChemCatChem, 2010, 2, 365 CrossRef CAS.
- J. Mondal, T. Sen and A. Bhaumik, Dalton Trans., 2012, 41, 6173 RSC.
- P. Wang, H. Liu, J. Niu, R. Li and J. Ma, Catal. Sci. Technol., 2014, 4, 1333 CAS.
- D. Beydoun, R. Amal, G. Low and S. McEvoy, J. Phys. Chem. B, 2000, 104, 4387 CrossRef CAS.
- D. Beydoun, R. Amal, G. Low and S. McEvoy, J. Mol. Catal. A: Chem., 2002, 180, 193 CrossRef CAS.
- W. L. Kostedt, J. Drwiega, D. W. Mazyck, S. W. Lee, W. Sigmund, C. Y. Wu and P. Chadik, Environ. Sci. Technol., 2005, 39, 8052 CrossRef CAS PubMed.
- Y.-L. Shi, W. Qiu and Y. Zheng, J. Phys. Chem. Solids, 2006, 67, 2409 CrossRef CAS.
- E. Al-Mutairi, K. Narasimharao and M. Mokhtar, RSC Adv., 2015, 5, 63917 RSC.
- E. Rafiee and S. Eavani, Green Chem., 2011, 13, 2112 RSC.
- E. Rafiee, S. Eavani and B. Malaekeh-Nikouei, Chem. Lett., 2012, 41, 438 CrossRef CAS.
- E. Rafiee and S. Eavani, J. Mol. Catal. A: Chem., 2013, 373, 30 CrossRef CAS.
- E. Rafiee, S. Eavani and M. Khodayari, Chin. J. Catal., 2013, 1513 CrossRef CAS.
- E. Rafiee, N. Rahpeima and S. Eavani, Acta Chim. Slov., 2014, 61, 177 CAS.
- E. Rafiee and N. Rahpeima, Chin. J. Catal., 2015, 36, 1342 CrossRef CAS.
- E. Rafiee, M. Kahrizi, M. Joshaghani and P. Ghaderi-Sheikhi Abadi, Res. Chem. Interm. DOI:10.1007/s11164-015-2387-5.
- E. Rafiee and S. Rezaei, J. Taiwan Inst. Chem. Eng., 2016, 61, 174 CrossRef CAS.
- E. Rafiee and M. Khodayari, J. Mol. Catal. A: Chem., 2015, 398, 336 CrossRef CAS.
- M. Hino and K. Arata, Chem. Lett., 1979, 477 CrossRef CAS.
- B. M. Reddya, P. M. Sreekanth, Y. Yamada and T. Kobayashi, J. Mol. Catal. A, 2005, 227, 81 CrossRef.
- D. J. Rosenberg, F. Coloma and J. A. Anderson, J. Catal., 2002, 210, 218 CrossRef CAS.
- Y. Xia, W. Hua and Z. Gao, Appl. Catal., A, 1999, 185, 293 CrossRef CAS.
- B. M. Reddy, P. M. Sreekanth, Y. Yamada, Q. Xu and T. Kobayashi, Appl. Catal., A, 2002, 228, 269 CrossRef CAS.
- Z. Gao, Y. Xia, W. Hua and C. Miao, Top. Catal., 1998, 6, 101 CrossRef CAS.
- D. Sawant-Dhuri, V. V. Balasubramanian, K. Ariga, D.-H. Park, J.-H. Choy, W. S. Cha, S. S. Al-deyab, S. B. Halligudi and A. Vinu, ChemCatChem, 2014, 6, 3347 CrossRef CAS.
- F. Su, Q. Wu, D. Song, X. Zhang, M. Wang and Y. Guo, J. Mater. Chem. A, 2013, 1, 13209 CAS.
- Y. Xuemin, D. Kai and M. Ping, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2015, 261 Search PubMed.
- R. Sheikh, G. N. Shao, Z. Khan, N. Abbas, H.-T. Kim and Y.-H. Park, Asia-Pac. J. Chem. Eng., 2015, 10, 339 CrossRef CAS.
- T. Kawakami, N. Tatsumi, M. Hirao and I. Hashiba, Jpn. Pat. Appl., H06-313188A, 1994.
- G. Vicente, A. Coteron, M. Martinez and J. Aracil, Ind. Crops Prod., 1998, 8, 29 CrossRef CAS.
- S. Furuta, H. Matsuhashi and K. Arata, Catal. Commun., 2004, 5, 721 CrossRef CAS.
- K. Suwannakarn, E. Lotero, J. G. Goodwin Jr and C. Lu, Appl. Catal., A, 2008, 255, 279 CAS.
- L. Bournay, D. Casanave, B. Delfort, G. Hillion and J. A. Chodorge, Catal. Today, 2005, 106, 190 CrossRef CAS.
- Q. Shu, B. Yang, H. Yuan, S. Qing and G. Zhu, Catal. Commun., 2007, 8, 2159 CrossRef CAS.
- A. Brito, M. E. Borges and N. Otero, Energy Fuels, 2007, 21, 3280 CrossRef CAS.
- T. Oku, T. Moriguchi and T. Akatsuka, Jpn. Pat. Appl., 2008-111085A, 2008.
- K. Nakajima, M. Hara and S. Hayashi, J. Am. Ceram. Soc., 2007, 90, 3725 CAS.
- L. Xu, Y. Wang, X. Yang, X. Yu, Y. Gao and J. H. Clark, Green Chem., 2008, 10, 746 RSC.
- N. Katada, T. Hatanaka, M. Ota, K. Yamada, K. Okumura and M. Niwa, Appl. Catal., A, 2009, 363, 164 CrossRef CAS.
- D. Sawant-Dhuri, V. V. Balasubramanian, K. Ariga, D.-H. Park, J.-H. Choy, W. S. Cha, S. S. Al-deyab, S. B. Halligudi and A. Vinu, ChemCatChem, 2014, 6, 3347 CrossRef CAS.
- S.-J. Yang, Y.-L. Xu, W.-P. Gong, Y.-K. Huang, G.-H. Wang, Y. Yang and C.-Q. Feng, Rare Met., 2015 DOI:10.1007/s12598-015-0521-6.
- S. R. Mukai, L. Lin, T. Masuda and K. Hashimoto, Chem. Eng. Sci., 2001, 56, 799 CrossRef CAS.
- S. R. Mukaia, M. Shimoda, L. Lin, H. Tamon and T. Masuda, Appl. Catal., A, 2003, 256, 107 CrossRef.
- W. Ruiping, G. Maiping and W. Jun, Chin. J. Chem. Eng., 2009, 17, 58 CrossRef.
- D. Jin, J. Gao, Z. Hou, Y. Guo, X. Lu, Y. Zhu and X. Zheng, Appl. Catal., A, 2009, 352, 259 CrossRef CAS.
- H. Aghayan, A. R. Mahjoub and A. R. Khanchi, Chem. Eng. J., 2013, 225, 509 CrossRef CAS.
- L. Yang, Y. Qi, X. Yuan, J. Shen and J. Kim, J. Mol. Catal., 2005, 229, 199 CrossRef CAS.
- J. Toufaily, M. Soulard and J. L. Guth, Colloids Surf., A, 2008, 316, 285 CrossRef CAS.
- C. Shi, R. Wang and G. Zhu, Eur. J. Inorg. Chem., 2005, 23, 4801 CrossRef.
- C. Shi, R. Wang and G. Zhu, Eur. J. Inorg. Chem., 2006, 15, 3054 CrossRef.
- B. C. Gagea, Y. Lorgouilloux, Y. Altintas, P. A. Jacobs and J. A. Martens, J. Catal., 2009, 265, 99 CrossRef CAS.
- B. Li, W. Ma, C. Han, J. Liu, X. Pang and X. Gao, Microporous Mesoporous Mater., 2012, 156, 73 CrossRef CAS.
- T. Chen, Y.-C. Che, Y. Zhang, J.-M. Zhang, F. Wang and Z.-Z. Wang, J. Porous Mater., 2014, 21, 495 CrossRef CAS.
- C.-Y. Sun, S.-X. Liu, D.-D. Liang, K.-Z. Shao, Y.-H. Ren and Z.-M. Su, J. Am. Chem. Soc., 2009, 131, 1883 CrossRef CAS PubMed.
- L. H. Wee, N. Janssens, S. R. Bajpe, C. E. A. Kirschhock and J. A. Martens, Catal. Today, 2011, 171, 275 CrossRef CAS.
- E. Rafiee and N. Nobakht, J. Mol. Catal. A: Chem., 2015, 398, 17 CrossRef CAS.
- E. Rafiee and N. Nobakht, Korean J. Chem. Eng., 2016, 33, 132 CrossRef CAS.
- J. Song, Z. Luo, D. K. Britt, H. Furukawa, O. M. Yaghi, K. I. Hardcastle and C. L. Hill, J. Am. Chem. Soc., 2011, 133, 16839 CrossRef CAS PubMed.
- H. Yang, J. Li, L. Wang, W. Dai, Y. Lv and S. Gao, Catal. Commun., 2013, 35, 101 CrossRef CAS.
- H. Yang, J. Li, H. Zhang, Y. Lv and S. Gao, Microporous Mesoporous Mater., 2014, 195, 87 CrossRef CAS.
- Q. Han, C. He, M. Zhao, B. Qi, J. Niu and C. Duan, J. Am. Chem. Soc., 2013, 135, 10186 CrossRef CAS PubMed.
- H. Wan, C. Chen, Z. Wu, Y. Que, Y. Feng, W. Wang, L. Wang, G. Guan and X. Liu, ChemCatChem, 2015, 7, 441 CrossRef CAS.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040 CrossRef PubMed.
- S. Ribeiro, A. D. S. Barbosa, A. C. Gomes, M. Pillinger, I. S. Gonçalves, L. Cunha-Silva and S. S. Balula, Fuel Process. Technol., 2013, 116, 350 CrossRef CAS.
- A. E. R. S. Khder, H. M. A. Hassan and M. S. El-Shall, Appl. Catal., A, 2014, 487, 110 CrossRef CAS.
- L. Bromberg and T. Alan Hatton, ACS Appl. Mater. Interfaces, 2011, 3, 4756 CAS.
- L. H. Wee, F. Bonino, C. Lamberti, S. Bordiga and J. A. Martens, Green Chem., 2014, 16, 1351 RSC.
- D.-Y. Du, J.-S. Qin, S.-L. Li, Z.-M. Su and Y.-Q. Lan, Chem. Soc. Rev., 2014, 43, 4615 RSC.
- I. Ahmed, N. A. Khan, Z. Hasan and S. H. Jhung, J. Hazard. Mater., 2013, 250–251, 37 CrossRef CAS PubMed.
- E. V. Ramos-Fernandez, C. Pieters, B. van der Linden, J. Juan-Alcañiz, P. Serra-Crespo, M. W. G. M. Verhoeven, H. Niemantsverdriet, J. Gascon and F. Kapteijn, J. Catal., 2012, 289, 42 CrossRef CAS.
- N. V. Maksimchuk, M. N. Timofeeva, M. S. Melgunov, A. N. Shmakov, Y. A. Chesalov, D. N. Dybtsev, V. P. Fedin and O. A. Kholdeeva, J. Catal., 2008, 257, 315 CrossRef CAS.
- S. Ribeiro, C. M. Granadeiro, P. Silva, F. A. A. Paz, F. F. de Biani, L. Cunha-Silvaa and S. S. Balula, Catal. Sci. Technol., 2013, 3, 2404 CAS.
- C. M. Granadeiro, A. D. S. Barbosa, S. Ribeiro, I. C. M. S. Santos, B. de Castro, L. Cunha-Silva and S. S. Balula, Catal. Sci. Technol., 2014, 4, 1416 CAS.
- D. Julião, A. C. Gomes, M. Pillinger, L. Cunha-Silva, B. de Castro, I. S. Gonçalves and S. S. Balula, Fuel Process. Technol., 2015, 131, 78 CrossRef.
- X. Hu, Y. Lu, F. Dai, C. Liu and Y. Liu, Microporous Mesoporous Mater., 2013, 170, 36 CrossRef CAS.
- J. Juan-Alcañiz, E. V. Ramos-Fernandez, U. Lafont, J. Gascon and F. Kapteijn, J. Catal., 2010, 269, 229 CrossRef.
- M. Ammar, S. Jiang and S. Ji, J. Solid State Chem., 2016, 233, 303 CrossRef CAS.
- S. Li, L. Zhang, K. P. O'Halloran, H. Ma and H. Pang, Dalton Trans., 2015, 44, 2062 RSC.
- X. Wang, X. Rong, H. Lin, J. Cao, G. Liu and Z. Chang, Inorg. Chem. Commun., 2016, 63, 30 CrossRef CAS.
- G. Peng, Y. Wang, C. Hu, E. Wang, S. Feng, Y. Zhou, H. Ding and Y. Liu, Appl. Catal., A, 2001, 218, 91 CrossRef CAS.
- D. Li, Y. Guo, C. Hu, L. Mao and E. Wang, Appl. Catal., A, 2002, 235, 11 CrossRef CAS.
- Y. Guo, Y. Yang, C. Hu, C. Guo, E. Wang, Y. Zou and S. Feng, J. Mater. Chem., 2002, 12, 3046 RSC.
- D. Li, Y. Guo, C. Hu, L. Mao and E. Wang, Chin. Chem. Lett., 2002, 13, 575 CAS.
- B. Li, Z. Liu, J. Liu, Z. Zhou, X. Gao, X. Pang and H. Sheng, J. Colloid Interface Sci., 2011, 362, 450 CrossRef CAS PubMed.
- J. Dou and H. C. Zeng, J. Am. Chem. Soc., 2012, 134, 16235 CrossRef CAS PubMed.
- T. Okada, K. Miyamoto, T. Sakai and S. Mishima, ACS Catal., 2014, 4, 73 CrossRef CAS.
- L. Xu, X. Yang, Y. Guo, F. Ma, Y. Guo, X. Yuan and M. Huo, J. Hazard. Mater., 2010, 178, 1070 CrossRef CAS PubMed.
- L. Li, Y. Li, Y. Ma and Y. Guo, J. Rare Earths, 2007, 25, 68 CrossRef.
- J. Li, W. Kang, X. Yang, X. Yu, L. Xu, Y. Guo, H. Fang and S. Zhang, Desalination, 2010, 255, 107 CrossRef CAS.
- K. Li, X. Yang, Y. Guo, F. Ma, H. Li, L. Chen and Y. Guo, Appl. Catal., B, 2010, 99, 364 CrossRef CAS.
- K. Li, Y. Guo, F. Ma, H. Li, L. Chen and Y. Guo, Catal. Commun., 2010, 11, 839 CrossRef CAS.
- M. Blanco and L. Pizzio, Appl. Catal., A, 2011, 405, 69 CrossRef CAS.
- V. Fuchs, L. Mendez, M. Blanco and L. Pizzio, Appl. Catal., A, 2009, 358, 73 CrossRef CAS.
- M. N. Blanco and L. R. Pizzio, Appl. Surf. Sci., 2010, 256, 3546 CrossRef CAS.
- V. M. Fuchs, E. L. Soto, M. N. Blanco and L. R. Pizzio, J. Colloid Interface Sci., 2008, 327, 403 CrossRef CAS PubMed.
- S. Jiang, Y. Guo, C. Wang, X. Qu and L. Li, J. Colloid Interface Sci., 2007, 308, 208 CrossRef CAS PubMed.
- L. Xu, Y. Wang, X. Yang, X. Yu, Y. Guo and J. H. Clark, Green Chem., 2008, 10, 746 RSC.
- C. Jiang, Y. Guo, C. Hu, C. Wang and D. Li, Mater. Res. Bull., 2004, 39, 251 CrossRef CAS.
- S. Farhadi and M. Zaidi, Appl. Catal., A, 2009, 354, 119 CrossRef CAS.
- S. Farhadi and Z. Momeni, J. Mol. Catal. A: Chem., 2007, 277, 47 CrossRef CAS.
- S. Farhadi and S. Sepahvand, J. Mol. Catal. A: Chem., 2010, 218, 75 CrossRef.
- G. S. Armatas, G. Bilis and M. Louloudi, J. Mater. Chem., 2011, 21, 2997 RSC.
- G. S. Armatas, A. P. Katsoulidis, D. E. Petrakis, P. J. Pomonis and M. G. Kanatzidis, Chem. Mater., 2010, 22, 5739 CrossRef CAS.
- I. Tamiolakis, I. N. Lykakis, A. P. Katsoulidis, M. Stratakis and G. S. Armatas, Chem. Mater., 2011, 23, 4204 CrossRef CAS.
- H. Li, N. Perkas, Q. Li, Y. Gofer, Y. Koltypin and A. Gedanken, Langmuir, 2003, 19, 10409 CrossRef CAS.
- A. M. Liu, K. Hidajat, S. Kawi and D. Y. Zhao, Chem. Commun., 2000, 1145 RSC.
- S. Araki, H. Doi, Y. Sano, S. Tanaka and Y. Miyake, J. Colloid Interface Sci., 2009, 339, 382 CrossRef CAS PubMed.
- M. Pirouzmand, M. M. Amini and N. Safari, J. Colloid Interface Sci., 2008, 319, 199 CrossRef CAS PubMed.
- S. W. Song, K. Hidajat and S. Kawi, Langmuir, 2005, 21, 9568 CrossRef CAS PubMed.
- N. Ren, A. G. Dong, W. B. Cai, Y. H. Zhang, W. L. Yang, S. J. Huo, Y. Chen, S. H. Xie, Z. Gao and Y. Tang, J. Mater. Chem., 2004, 14, 3548 RSC.
- T. Yokoi, T. Tatsumi and H. Yoshitake, J. Colloid Interface Sci., 2004, 274, 451 CrossRef CAS PubMed.
- A. S. M. Chong and X. S. Zhao, J. Phys. Chem. B, 2003, 107, 12650 CrossRef CAS.
- M. Gil, I. Tiscornia, O. de la Iglesia, R. Mallada and J. Santamaria, Chem. Eng. J., 2011, 175, 291 CrossRef CAS.
- J. W. Wei, J. J. Shi, H. Pan, Q. F. Su, J. B. Zhu and Y. Shi, Microporous Mesoporous Mater., 2009, 117, 596 CrossRef CAS.
- L. Zhao, Y. Chi, Q. Yuan, N. Li, W. Yan and X. Li, J. Colloid Interface Sci., 2013, 390, 70 CrossRef CAS PubMed.
- B. J. S. Johnson and A. Stein, Inorg. Chem., 2001, 40, 801 CrossRef CAS PubMed.
- B. J. S. Johnson and A. Stein, J. Colloid Interface Sci., 2011, 360, 189 CrossRef PubMed.
- H.-l. Li, N. Perkas, Q.-l. Li, Y. Gofer, Y. Koltypin and A. Gedanken, Langmuir, 2003, 19, 10409 CrossRef CAS.
- G. W. Brindley and G. Brown, Crystal structures of clay minerals and their X-ray identification, Mineralogical Society, England, 1980 Search PubMed.
- A. J. López, J. M. Rodriguez, E. R. Castellon and P. O. Pastor, J. Mol. Catal. A: Chem., 1996, 108, 175 CrossRef.
- D. M. Moore and R. C. Reynolds Jr, X-ray diffraction and the identification and analysis of clay minerals, Oxford, New York, second edn, 1997 Search PubMed.
- M. Suárez and E. García-Romero, Appl. Clay Sci., 2006, 31, 154 CrossRef.
- J. L. Cao, G. S. Shao, Y. Wang, Y. Liu and Z. Y. Yuan, Catal. Commun., 2008, 9, 2555 CrossRef CAS.
- L. B. de Paiva, A. R. Morales and F. R. Valenzuela Díaz, Appl. Clay Sci., 2008, 42, 8 CrossRef.
- S. R. Ha and K. Y. Rhee, Colloids Surf., A, 2008, 322, 1 CrossRef CAS.
- J. H. Huang, Y. F. Liu and X. G. Wang, J. Mol. Catal. B: Enzym., 2008, 57, 10 CrossRef.
- C. Pereira, A. R. Silva, A. P. Carvalho, J. Pires and C. Freire, J. Mol. Catal. A: Chem., 2008, 283, 5 CrossRef CAS.
- L. Zhang, Q. Jin, L. Shan, Y. Liu, X. Wang and J. Huang, Appl. Clay Sci., 2010, 47, 229 CrossRef CAS.
- X. Zheng, L. Zhang, J. Li, S. Luo and J.-P. Cheng, Chem. Commun., 2011, 47, 12325 RSC.
- M. Cohen and R. Neumann, J. Mol. Catal. A: Chem., 1999, 146, 291 CrossRef CAS.
- R. Tayebee, M. M. Amini, M. Akbari and A. Aliakbari, Dalton Trans., 2015, 44, 9596 RSC.
- H. Kim, J. C. Jung, S. H. Yeom, K. Y. Lee, J. Yi and I. K. Song, Mater. Res. Bull., 2007, 42, 2132 CrossRef CAS.
- R. J. Errington, S. S. Petkar, B. R. Horrocks, A. Houlton, L. H. Lie and S. N. Patole, Angew. Chem., Int. Ed., 2005, 44, 1254 CrossRef CAS PubMed.
- K. N. Rao, A. Sridhar, A. F. Lee, S. J. Tavener, N. A. Young and K. Wilson, Green Chem., 2006, 8, 790 RSC.
- R. Zhang and C. Yang, J. Mater. Chem., 2008, 18, 2691 RSC.
- M. Lu, W. M. Nolte, T. He, D. A. Corley and J. M. Tour, Chem. Mater., 2009, 21, 442 CrossRef CAS.
- E. Grinenval, X. Rozanska, A. Baudouin, E. Berrier, F. Delbecq, P. Sautet, J. M. Basset and F. Lefebvre, J. Phys. Chem. C, 2010, 114, 19024 CAS.
- E. Grinenval, J.-M. Basset and F. Lefebvre, J. Inorg. Chem., 2013 DOI:10.1155/2013/902192.
- E. Grinenval, F. Bayard, J.-M. Basset and F. Lefebvre, Inorg. Chem., 2014, 53, 2022 CrossRef CAS PubMed.
- Y. Xiao, D. Chen, N. Ma, Z. Hou, M. Hu, C. Wang and W. Wang, RSC Adv., 2013, 3, 21544 RSC.
- X. Chen, H. Li, P. Yin and T. Liu, Chem. Commun., 2015, 51, 6104 RSC.
- O. A. Kholdeeva, N. V. Maksimchuk and G. M. Maksimov, Catal. Today, 2010, 157, 107 CrossRef CAS.
- Z. Karimi, A. R. Mahjoub and S. M. Harati, Inorg. Chim. Acta, 2011, 376, 1 CrossRef CAS.
- Y. Guo and C. Hu, J. Mol. Catal. A: Chem., 2007, 262, 136 CrossRef CAS.
- Y. Guo, C. Hu, C. Jiang, Y. Yang, S. Jiang, X. Li and E. Wang, J. Catal., 2003, 217, 141 CAS.
- H.-J. Kim, Y.-G. Shul and H. Han, Appl. Catal., A, 2006, 299, 46 CrossRef CAS.
- S. Farhadi, M. Afshari, M. Maleki and Z. Babazadeh, Tetrahedron Lett., 2005, 46, 8483 CrossRef CAS.
- A. Tarlani, M. Abedini, A. Nemati, M. Khabaz and M. M. Amini, J. Colloid Interface Sci., 2006, 303, 32 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |