Three-dimensional porous carbon nanosheet networks anchored with Cu6Sn5@carbon as a high-performance anode material for lithium ion batteries†
Zhiyuan Wang*ab,
Shaohua Luoab,
Fang Chena,
Dan Wangab,
Yanguo Liuab,
Xiwei Qiab,
Chunsheng Shic and
Naiqin Zhaoc
aSchool of Resources and Materials, Northeastern University at Qinhuangdao, Qinhuangdao 066004, China. E-mail: zhiyuanwang@neuq.edu.cn; luosh@neuq.edu.cn
bKey Laboratory of Dielectric and Electrolyte Functional Material Hebei Province, Qinhuangdao, China
cSchool of Materials Science and Engineering, Tianjin University, Tianjin 300072, P. R. China
First published on 3rd June 2016
Abstract
The poor cycling stability resulting from large volume change is the major obstacle to the application of tin-based anode materials. In this paper, three-dimensional porous carbon nanosheet networks anchored with Cu6Sn5@carbon nanoparticles (10–35 nm) as a high-performance anode for lithium ion batteries are synthesized via a self-assembly NaCl template-assisted in situ chemical vapor deposition strategy. The composite exhibits superior rate capability (523, 443, 395, 327, 281, and 203 mA h g−1 at 0.2, 0.5, 1, 2, 5, and 10 A g−1, respectively) and excellent cycling stability (396.8 mA h g−1 at 1 A g−1 for the first cycle and maintains 92.3% after 200 cycles). The superior performance is attributed to the unique architecture: inactive metal copper serves as a “buffer matrix” and relaxes the large volume change of the tin; a uniform distribution of nano-sized Cu6Sn5 makes the inevitable stress/strain small, meanwhile it provides a short path for lithium ion diffusion; onion-like carbon shells not only prevent the Cu6Sn5 nanoparticles from agglomerating and growing but also offer mechanical support to accommodate the stress associated with the volume change of tin upon cycling, thus alleviating pulverization; 3D porous carbon nanosheet networks ensure the mechanical integrity and facilitate lithium ion diffusion as well as electron transportation.
Introduction
Tin is considered to be a suitable candidate for anode materials of next generation Li-ion batteries (LIBs) due to its high theoretical gravimetric capacity (994 mA h g−1) and volumetric capacity (7246 mA h cm−3), compared with the commercial graphite anode1 (372 mA h g−1 and 837 mA h cm−3). However, its large volume change (up to 260%) associated with the insertion and extraction of lithium leads to electrode pulverization, resultant loss of contact with the current collector, severe particle aggregation, and continual formation of a very thick solid electrolyte interphase (SEI) on the Sn surfaces upon cycling, thereby resulting in rapid capacity fading and poor cycling stability.2,3Recently, extensive efforts have been made to address the issues posed by the volume change of Sn and to improve its electrochemical performance. The three most effective approaches are summarized as follows. First, reducing Sn particle size to nanoscale can smaller stress/strain over the entire electrode during lithiation/delithiation and prevent local cracking. In this context, Sn-based nanomaterials with different nanostructures, including nanoparticles,4,5 nanowires/nanotubes,6,7 nanosheets/nanoplates,8,9 and 3D nanoarchitectures10,11 have been explored and their electrochemical performances are apparently superior to that of the bulk materials. Second, dispersing nano-sized Sn into a conductive matrix (such as carbon materials) can accommodate volume change, maintain mechanical integrity, and enhance conductivity of the composite electrode. So nanostructured Sn–C composites with Sn nanoparticles uniformly dispersed in a supporting matrix have been synthesized to enhance the electrochemical performance.12–17 For instance, Bruno Scrosati reported that nanostructured Sn–C composite delivered a specific capacity of 500 g−1 and remained stable over more than 200 cycles.12 Recently, we reported a graphene network anchored with Sn@graphene synthesized by in situ chemical vapor deposition (CVD) technique, and it demonstrated excellent electrochemical performance.14 Third, using intermetallic alloy containing both active and inactive elements can relieve volume change.18 In such alloy anodes, active metal reacts with lithium to increase capacity, and inactive metal serves as “buffer matrix” and relieves the volume change of the active phase to some extent. Han synthesized monodisperse nanospheres of intermetallic M–Sn (M = Fe, Co, Cu, and Ni) phases with improved electrochemical performance.3
In summary, significant improvements in electrochemical performances of Sn anode have been achieved by above modifications. However, any single strategy cannot overcome the volume change problem completely. It is expected a method that integrate those three approaches could construct a stable uniform composite structure which formed by nano-sized Sn-based alloy uniformly embedded in a conductive carbon matrix will alleviate volume change effectively and achieve an excellent cycling stability. Among all the alternative metals that can form intermetallic alloy with Sn, such as nickel,19–21 stibium,22–24 iron,25–27 cobalt,28–30 and copper,31–35 copper is remarkable because of its high conductivity and elasticity. Xia reported a reduced graphene oxide (RGO)/Cu6Sn5 composite in which nano-sized Cu6Sn5 was sandwiched between multilayer graphene sheets. The composite with graphene network displayed better electrochemical performance than the Cu6Sn5 nanoparticles anode.36 Yang also fabricated graphene/Cu6Sn5 nanocomposite anode with enhanced electrochemical performance.37 Unfortunately, the Cu6Sn5 in the as-prepared samples exhibit a disordered morphology, size, and distribution, which is largely impeded by the synthesis process and very difficult to be tailored. Moreover, both of two methods are very costly, time and energy consuming due to the tedious preparation of reduced graphene oxide in advance.
Obviously, intermetallic alloy, nanoparticle size, uniform distribution, and porous conductive network structure are the key factors for tin-based materials to achieve high electrochemical performance. However, it is a challenge to fabricate such tin-based composite with all the above features and the related report is rare. Herein, we report a facile and scalable strategy to synthesize such composite of 3D porous carbon nanosheet networks anchored with Cu6Sn5@carbon (3D PCNNWs-Cu6Sn5@C) as an advanced anode material for high performance LIBs. 3D porous carbon nanosheet networks are synthesized via a simple freeze-drying process using the self-assembly cubic NaCl salt as a sacrificial template and Cu6Sn5@carbon with 10–35 nm in diameter are homogeneously embedded in the 3D porous carbon networks. It is expected that the unique structure will display an outstanding electrochemical performance including large reversible capacity, high coulombic efficiency, excellent cyclic performance, and good rate capability.
Experimental section
Synthesis of 3D PCNNWs-Cu6Sn5@C
The synthesis of 3D PCNNWs-Cu6Sn5@C consisted of solution preparation, freeze drying, and CVD process. All the reagents were purchased from Sinopharm Chemical Reagent Co.,Ltd and used without further purification. Citric acid (2.5 g), SnCl2·2H2O (0.185 g), Cu(NO3)3·9H2O (0.234 g) and NaCl (20.7 g) were dissolved in deionized water (75 mL) by magnetic stirring. The obtained solution was frozen in a refrigerator at −20 °C for 24 h. After that the resulting gel was put in a freeze drier to eliminate the water completely and then was ground to a fine composite powder. Then 20 g composite powder was put in a quartz boat, annealed at 750 °C for 2 h under mixed gas H2 and Ar (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), and cooled to room temperature under the protection gas. Finally, the as-synthesized product was washed with deionized water several times to remove NaCl, and then pure 3D PCNNWs-Cu6Sn5@C was obtained. For comparison, Cu6Sn5 particles embedded in amorphous carbon (Cu6Sn5/carbon composite) were also prepared at the same conditions by carbonizing the mixture of Cu(NO3)3·9H2O, SnCl2, and citric acid without NaCl as template.
3), and cooled to room temperature under the protection gas. Finally, the as-synthesized product was washed with deionized water several times to remove NaCl, and then pure 3D PCNNWs-Cu6Sn5@C was obtained. For comparison, Cu6Sn5 particles embedded in amorphous carbon (Cu6Sn5/carbon composite) were also prepared at the same conditions by carbonizing the mixture of Cu(NO3)3·9H2O, SnCl2, and citric acid without NaCl as template.
Physical characterization
XRD pattern was obtained from an X-ray diffractometer (Rigaku Smartlab) with Cu Kα radiation (λ = 0.15406 nm) in the 2θ range from 10° to 80°. The morphology was examined by a field emission scanning electron microscope (SEM, ZEISS SUPRA55) and a high-resolution transmission electron microscope (HRTEM, JEM 2100F). Nitrogen adsorption and desorption isotherms of the as-prepared material were measured at 77 K using an autosorb iQ instrument (Quantachrome U.S.). The total surface area was calculated by the Brunauer–Emmett–Teller (BET) method, and the pore size distribution was calculated by the Density Functional Theory (DFT) method based on the adsorption and desorption data. The content of Cu6Sn5 in the composite was measured by the thermogravimetry and differential thermal analysis (TG/DTA) (Hengjiu Instrument Co., Ltd., HCT-2, Beijing, China) from room temperature to 1000 °C at a rate of 10 °C min−1 in air. A micro-Raman spectrometer (Renishaw, InVia microscope) with a 532 nm laser was used in the Raman study to analyze the characteristics of carbon in the composite.Electrochemical measurements
To evaluate the electrochemical performance of 3D PCNNWs-Cu6Sn5@C and Cu6Sn5/carbon composite, the composites were assembled in a CR 2032-type coin cell. First, the synthesized materials were mixed with carbon black and polyvinylidene fluoride in N-methyl-2-pyrrolidinone solvent with a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. After stirring for 4 h, the slurry was coated on a copper foil, dried at 120 °C overnight in a vacuum oven and cut into a wafer with area of 0.785 cm2, the loading amount is about 1.5–2 mg cm−2 for the electrode, coin cell was assembled in an argon-filled glove box where the lithium plays as the counter electrode and reference electrode, Celgard 2300 polypropylene film as separator and 1 M LiPF6 dissolving in ethylene carbonate/ethyl methyl carbonate/dimethyl carbonate (1
10. After stirring for 4 h, the slurry was coated on a copper foil, dried at 120 °C overnight in a vacuum oven and cut into a wafer with area of 0.785 cm2, the loading amount is about 1.5–2 mg cm−2 for the electrode, coin cell was assembled in an argon-filled glove box where the lithium plays as the counter electrode and reference electrode, Celgard 2300 polypropylene film as separator and 1 M LiPF6 dissolving in ethylene carbonate/ethyl methyl carbonate/dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) as electrolyte. Galvanostatic charge/discharge tests were performed using a battery test system (CT2001A, Wuhan LAND Electronic Co., Ltd., China) in a voltage range of 0.005–3 V with different current densities. The cyclic voltammetry (CV) test was conducted by an electrochemical workstation (Solartron 1260 + 1287) between 0.005 and 3 V versus Li+/Li at a scan rate of 0.1 mV s−1 at room temperature. The electrochemical impedance spectroscopy (EIS) measurement was performed through the same electrochemical workstation with an amplitude voltage of 5 mV and frequency range of 10 mHz–100 kHz.
1 in volume) as electrolyte. Galvanostatic charge/discharge tests were performed using a battery test system (CT2001A, Wuhan LAND Electronic Co., Ltd., China) in a voltage range of 0.005–3 V with different current densities. The cyclic voltammetry (CV) test was conducted by an electrochemical workstation (Solartron 1260 + 1287) between 0.005 and 3 V versus Li+/Li at a scan rate of 0.1 mV s−1 at room temperature. The electrochemical impedance spectroscopy (EIS) measurement was performed through the same electrochemical workstation with an amplitude voltage of 5 mV and frequency range of 10 mHz–100 kHz.
Results and discussion
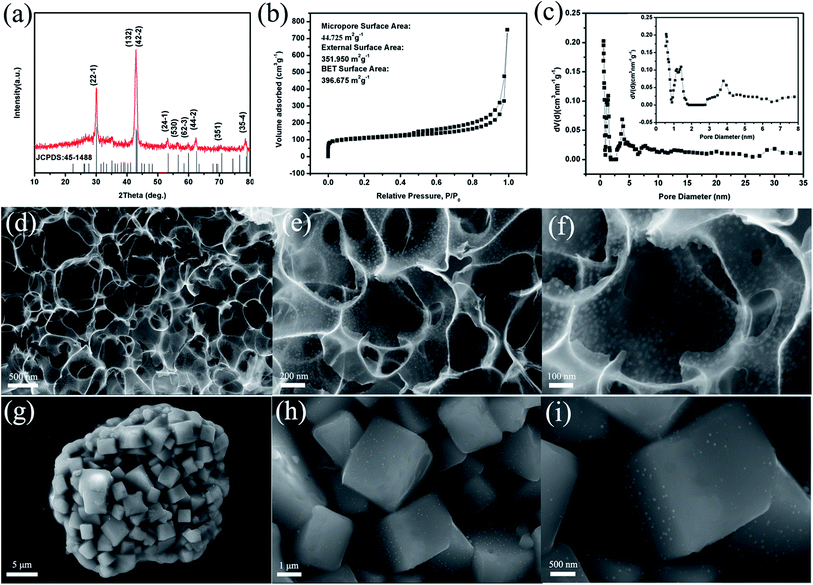
The fabrication process of 3D PCNNWs-Cu6Sn5@C is schematically depicted in Fig. 1. The entire fabrication process consists of three main steps. Firstly, the raw materials citric acid, SnCl2·2H2O, Cu(NO3)3·9H2O and NaCl in a certain proportion were dissolved in deionized water to form solution. Herein, SnCl2·2H2O and Cu(NO3)3·9H2O were used as the precursor to form Cu6Sn5 alloy. Citric acid, a weak organic acid containing three carboxyl groups, formed complexation with metal ions and inhibited the hydrolysis of Sn2+ and Cu3+; meanwhile, it is used as precursors to synthesize three-dimensional porous carbon nanosheet networks through dehydration and carbonization. Secondly, the solution was freeze dried. During this process, the NaCl grew into cubes and further self-assembled into three-dimensional structure, the mixture (citric acid, SnCl2·2H2O, Cu(NO3)·9H2O) covered on the surface of the self-assembled 3D NaCl. Finally, the precursor reacted under chemical vapor deposition (CVD) conditions and 3D macroporous graphene networks anchored with Cu6Sn5 nanoparticles directly grown on the scaffold of self-assembled NaCl template and then the NaCl skeleton was removed by washing with water, which left the free-standing 3D PCNNWs-Cu6Sn5@C, as displayed in Fig. 1 and 2d–i.X-ray diffraction (XRD) measurement was performed to determine the crystal structure of the as-prepared product (3D PCNNWs-Cu6Sn5@C). As shown in Fig. 2a, all the peaks in the pattern can be well aligned with a monoclinic Cu6Sn5 crystal structure (JCPDS no. 45-1488), and no other characteristic peaks of Sn, Cu or SnO2 is observed in XRD pattern, which implies complete formation of the targeted alloy (Cu6Sn5) from SnCl2 and Cu(NO3)2 by annealing under the protection gas according to the Cu–Sn phase diagram (Fig. S1†). And the sharpness of the diffraction peaks indicates that the Cu6Sn5 phase in the composite is well crystallized. The average particle size of Cu6Sn5 calculated from the largest low-angle diffraction peak (22![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) according to the Scherrer equation is about 25.9 nm, which is well consistent with the average diameter (24 nm) revealed by the TEM analysis. To determine the content of Cu6Sn5 in the 3D PCNNWs-Cu6Sn5@C composite, TG–DTA was carried out in air at a heating rate of 10 °C min−1 (Fig. S2†). The sample was heated to 1000 °C and kept for 30 min so that Cu6Sn5 alloy could be oxidized to CuO and SnO2, and carbon could be oxidized to CO2 completely. According to the eqn (S1) (ESI†), the Cu6Sn5 is calculated to be 46.4% based on the remaining weight (CuO and SnO2) and the TG–DTA curves.
) according to the Scherrer equation is about 25.9 nm, which is well consistent with the average diameter (24 nm) revealed by the TEM analysis. To determine the content of Cu6Sn5 in the 3D PCNNWs-Cu6Sn5@C composite, TG–DTA was carried out in air at a heating rate of 10 °C min−1 (Fig. S2†). The sample was heated to 1000 °C and kept for 30 min so that Cu6Sn5 alloy could be oxidized to CuO and SnO2, and carbon could be oxidized to CO2 completely. According to the eqn (S1) (ESI†), the Cu6Sn5 is calculated to be 46.4% based on the remaining weight (CuO and SnO2) and the TG–DTA curves.
The morphology of the 3D PCNNWs-Cu6Sn5@C was investigated by scanning electron microscopy (SEM). As shown in Fig. 2d–f, the composite exhibits a unique interconnected 3D porous network appearing as a foam-like structure and many Cu6Sn5 nanoparticles with uniform particle size (10–35 nm) were homogenously anchored on the walls of porous carbon framework. The unique 3D porous carbon networks were obtained by using NaCl as a sacrificial template, which was demonstrated in our previous work.14 In Fig. 2d–i, we compared the SEM images of the CVD-synthesized product before and after eliminating NaCl, and found that the self-assembled 3D NaCl structure acts as a template for directing synthesis of the 3D porous carbon networks as well as plays a role of preventing the growth and aggregation of Cu6Sn5 nanoparticles during the CVD process, resulting in the formation of small and uniform Cu6Sn5 nanoparticles with high catalytic activity. The Cu6Sn5 nanoparticles can catalyze the growth of onion-like carbon shells around them, as demonstrated in the TEM characterization. After washing with water to remove the NaCl, the as-prepared sample is supposed to be a close-packed uniform cubic empty “cells” of 1–2 μm in side length, which consists of carbon nanosheets anchored with Cu6Sn5 nanoparticles. However, it is obvious that the close-packed uniform the cubic empty “cells” transformed to irregular interconnected 3D porous networks after drying because the ultrathin carbon walls turned into curved caused by van der Waals forces during drying (see Fig. 2d–f). Furthermore, the size of the interconnected macropores was approximately the same as the size of cubic NaCl particle, which confirms the role of NaCl as the template for growing 3D porous structure. Such an interconnected 3D porous network is beneficial for penetration of electrolyte and mechanical stability of the electrode.
Nitrogen adsorption–desorption measurement was carried out to further investigate the porous structure of 3D PCNNWs-Cu6Sn5@C, as illustrated in Fig. 2b. The as-prepared sample exhibits a type-IV isotherm with a large hysteresis loop, which can be ascribed to a mesoporous structure with a large amount of uniform pores, as reported previously.38 The Brunauer–Emmett–Teller (BET) surface area of the 3D PCNNWs-Cu6Sn5@C is measured to be 396 m2 g−1. Pore size distribution was calculated by the Density Functional Theory (DFT) method based on the adsorption and desorption data, as displayed in Fig. 2c. The total pore volume is 0.576 cm3 g−1 and a uniform micropores distribution with an average size of 1.2 nm is determined by DFT analysis curve (inset of Fig. 2c). The micropores maybe come from the carbon nanoshells outside Cu6Sn5 nanoparticles. Furthermore, a broad peak at around 3.8 nm is found for the 3D PCNNWs-Cu6Sn5@C, corresponding to the mesopores among the clusters (Fig. 2c). The above results demonstrate the 3D carbon networks have a large number of pores and a high surface area. Porous structure is favourable to electrolyte ions diffusion and large surface area provides more active sites for Li+ storage. Meanwhile, 3D carbon networks can offer more freedom space to accommodate the large volume change of Cu6Sn5 nanoparticles during insertion and extraction of lithium ion.
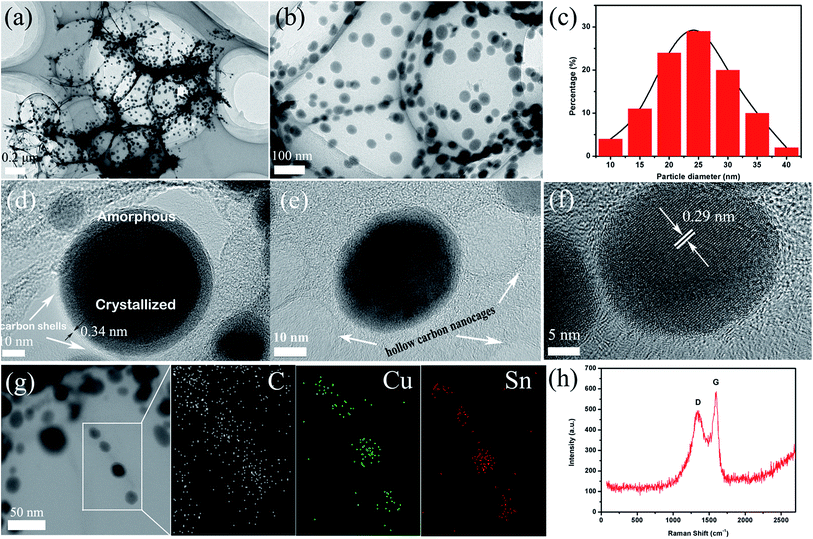
To better investigate the microstructure and morphology of 3D PCNNWs-Cu6Sn5@C, the as-obtained sample was further observed using TEM and HRTEM. Fig. 3a and b presents the typical TEM images of 3D PCNNWs-Cu6Sn5@C, it is obvious that the sample exhibits a 3D porous ultrathin architecture with many black Cu6Sn5 nanoparticles homogeneously embedded in the carbon nanosheets. The nanosheets exhibit a curved characteristic with a low contrast, implying ultrathin carbon nanosheets (less than 30 nm). Particle size analysis (Fig. 3c) displays a narrow distribution and an average diameter of 24 nm. The lattice fringe in the HRTEM image (Fig. 3f) reveals a clear lattice spacing of 0.29 nm, in good agreement with that of the (22![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) plane of Cu6Sn5 (JCPDS 45-1488). The scanning TEM elemental mapping (Fig. 3g) further remarkably reveals that the alloy elements (Cu and Sn) are uniformly distributed in the carbon nanosheets. What's more, these Cu6Sn5 nanoparticles are entirely encapsulated in thin onion-like carbon shells on the graphitic carbon nanosheets (Fig. 3d). The interplanar spacing for the shells is 0.34 nm, corresponding to (002) planes of graphite. The ordered graphitized carbon nanoshells outside the Cu6Sn5 seems to be induced by the Cu6Sn5 nanoparticles during the heating process. As a result, carbon nanoshells can prevent Cu6Sn5 nanoparticles from agglomerating and growing, as well as provide mechanical support to accommodate the stress associated with the volume change of nano-Sn upon prolonged cycling, thus it can alleviates electrode pulverization. Interestingly, when the sample was prepared for TEM observation and suffered from long-time intense ultrasonication, we found only a few of the embedded Cu6Sn5 particles were removed by the ultrasonication and some hollow carbon nanocages were remained (Fig. 3e). Most of the Cu6Sn5 nanoparticles are still firmly immobilized on the carbon nanosheets (Fig. 3a and b), indicating the strong connection between Cu6Sn5@C nanoparticles and carbon matrix. Moreover, when the Cu6Sn5 nanoparticles were further observed in detail by HRTEM (Fig. 3d and e), we found that some Cu6Sn5 nanoparticles were not a uniform crystallized structure but exhibited a core–shell structure, in which the central part is highly crystallized, while the edge of the particle is amorphous. We inferred that the rapid cooling process during CVD might be the main reason for formation of this crystallized-amorphous core–shell structure of Cu6Sn5 nanoparticles.
) plane of Cu6Sn5 (JCPDS 45-1488). The scanning TEM elemental mapping (Fig. 3g) further remarkably reveals that the alloy elements (Cu and Sn) are uniformly distributed in the carbon nanosheets. What's more, these Cu6Sn5 nanoparticles are entirely encapsulated in thin onion-like carbon shells on the graphitic carbon nanosheets (Fig. 3d). The interplanar spacing for the shells is 0.34 nm, corresponding to (002) planes of graphite. The ordered graphitized carbon nanoshells outside the Cu6Sn5 seems to be induced by the Cu6Sn5 nanoparticles during the heating process. As a result, carbon nanoshells can prevent Cu6Sn5 nanoparticles from agglomerating and growing, as well as provide mechanical support to accommodate the stress associated with the volume change of nano-Sn upon prolonged cycling, thus it can alleviates electrode pulverization. Interestingly, when the sample was prepared for TEM observation and suffered from long-time intense ultrasonication, we found only a few of the embedded Cu6Sn5 particles were removed by the ultrasonication and some hollow carbon nanocages were remained (Fig. 3e). Most of the Cu6Sn5 nanoparticles are still firmly immobilized on the carbon nanosheets (Fig. 3a and b), indicating the strong connection between Cu6Sn5@C nanoparticles and carbon matrix. Moreover, when the Cu6Sn5 nanoparticles were further observed in detail by HRTEM (Fig. 3d and e), we found that some Cu6Sn5 nanoparticles were not a uniform crystallized structure but exhibited a core–shell structure, in which the central part is highly crystallized, while the edge of the particle is amorphous. We inferred that the rapid cooling process during CVD might be the main reason for formation of this crystallized-amorphous core–shell structure of Cu6Sn5 nanoparticles.
Raman spectroscopy was performed to further analyze the carbon material in the composite, as illustrated in Fig. 3h. The spectrum displays a D band at 1355 cm−1 and a G band at 1586 cm−1. It is reported that the D band is ascribed to sp3 carbon and defects such as topological defects, dangling bonds, and vacancies, while the G band is attributed to ordered sp2 carbon.14,39 Therefore, the ID/IG ratio represents the disorder degree of carbon material. In the Raman spectrum (Fig. 3h), the ID/IG ratio of 3D PCNNWs-Cu6Sn5@C is calculated to 0.85, which indicating that the carbon materials in the 3D networks are mainly composed of well-crystallized nanocrystalline graphite, in good agreement with HRTEM investigation. And the graphitic carbon not only enhances the conductivity of the composite but also offers more reversible active sites for lithium ions storage.
In the structure of 3D PCNNWs-Cu6Sn5@C, the uniform Cu6Sn5 nanoparticles make the inevitable stress/strain become small and uniform, and the copper in the Cu6Sn5 alloy can serve as “buffer matrix” to relax the volume change of Sn phase to some extent. The carbon shells confine the active Cu6Sn5 alloy within a closed volume so that the capacity fading due to pulverization and agglomeration can be minimized, the electric contact of active Cu6Sn5 with carbon nanosheets can be enhanced, and the side reaction for continuous formation of the solid electrolyte interphase (SEI) can be reduced. Furthermore, the assembled 3D carbon networks can facilitate electron transport and improve lithium ion diffusion within the composite.
As mentioned above, the unique structure may endow them with superior lithium ion storage performance. To confirm this supposition, the Li-ion insertion/extraction property of the composite was investigated by CR2032 coin cell. Fig. 4a shows cyclic voltammograms (CV) of 3D PCNNWs-Cu6Sn5@C for the initial three cycles at a sweep rate of 0.1 mV s−1 in the voltage range 0.005–3.0 V. Similar to the CV curves of Cu6Sn5 alloy previous reported,31,35 the cathodic part below 0.5 V is mainly ascribed to the stepwise formation process of Li3.5Sn alloy from Cu6Sn5 (corresponding to theoretical capacity 480 mA h g−1), agreeing well with the following discharge curves (Fig. 4b). During discharging at 0.5 V, Li+ insert into the crystal structure of Cu6Sn5 to form partially lithiated phase LixCu6Sn5 (eqn (1-1)). Further insertion of Li+ into LixCu6Sn5 leads to the formation of Li2CuSn at 0.3 V (eqn (1-2)), when the potential (<0.01 V) close to the metal lithium's, the lithiated compound Li2CuSn transforms into Li3.5Sn alloy (eqn (1-3)) that is surrounded by Cu matrix. The Cu in the Cu–Sn alloy seems to have no effect on lithiation/delithiation process since it is an inactive metal with Li. As a consequence, the Cu is beneficial to the electrochemical performance of the Cu6Sn5 alloy anode for its excellent electrical conductivity and buffering effect, which is demonstrated by the good rate capability and long cycle life observed in the electrochemical tests (Fig. 4c and e). Upon charging, multiple anodic humps located at around 0.56 V and 0.86 V are attributed to the de-alloying process, corresponding to the recovery of Li2CuSn from Li7Sn2 and Cu6Sn5 from Li2CuSn, respectively. The anodic peak at 1.26 V corresponds to the deintercalation of lithium ion from ultrathin carbon nanosheet networks.16 The overlapping of the curves for the second and third cycles indicates good reversibility of Li+ insertion/extraction during charging/discharging process.
| xLi + Cu6Sn5 → LixCu6Sn5 | (1-1) |
| (10 − x)Li + LixCu6Sn5 → 5Li2CuSn + Cu | (1-2) |
| 3Li + 2Li2CuSn → 2Li3.5Sn + 2Cu | (1-3) |
Fig. 4b presents the charge and discharge curves of 3D PCNNWs-Cu6Sn5@C for the initial three cycles at a current rate of 0.2 Ag−1. It exhibits a relatively smooth voltage profile due to the multi-step lithium ion intercalation reactions of Cu6Sn5, and the charge and discharge curves are nearly overlapping except for the first discharge curve, which also demonstrates a relatively excellent reversibility of Li+ insertion/extraction, in consistent with the CV results (Fig. 4a). As shown in Fig. 4b, the prepared sample delivers a discharge capacity of 893.8 mA h g−1 and a reversible capacity of 504.9 mA h g−1 in the first cycle. The reversible capacity is slightly higher than the maximum theoretical capacity of Cu6Sn5 alloy (480 mA h g−1). The exceed capacity may derive from three aspects: (i) Cu6Sn5 nanoparticles generate larger surface and interface for lithium storage; (ii) the 3D porous carbon nanosheet networks with high surface area provide more active sites for Li+, contributing to higher initial capacity; (iii) the unique structure of 3D PCNNWs-Cu6Sn5@C offer high structural stability during the first alloying process.
The cycle performance of 3D PCNNWs-Cu6Sn5@C and Cu6Sn5/carbon composite were evaluated by galvanostatic charging–discharging in a potential range of 0.005–3.0 V (vs. Li+/Li) at a current density of 1 A g−1 (Fig. 4e). The 3D PCNNWs-Cu6Sn5@C delivers a reversible capacity of 396.8 mA h g−1 for the first cycle and remains 366.4 mA h g−1 after 200 cycles with an outstanding capacity retention of 92.3%, which demonstrates that its structure is very stable during cycling. In contrast, the Cu6Sn5/carbon composite delivers a much lower reversible capacity (∼224 mA h g−1) and exhibits very fast capacity fading during cycling. Moreover, the average capacity value of the 3D PCNNWs-Cu6Sn5@C is higher than that of the previous reported Cu–Sn alloy and Cu6Sn5–carbon nanostructures tested at the same conditions (Table S1†),33,36,37 which is closely related to structure features: nano-sized Cu6Sn5, homogenous distribution, carbon nanoshells coating, and 3D carbon networks. However, the initial Coulomb efficiency of the prepared sample is only 56.5%, which is undesirable for practical application. Fortunately, the charge–discharge efficiency increases to 96.8% in the third cycle and remains over 98% till 200 cycles, which suggests a highly reversible lithium insertion/extraction associated with fast electron transport in the electrodes. The low initial coulomb efficiency is mainly due to the irreversible processes that the ‘rearrangement’ of the electrode structure and some active sites (disorder structure) become inactive, resulting in dead lithium. To identify if the unique architecture is stable after the repeated cycling, we investigated the morphology and structure of 3D PCNNWs-Cu6Sn5@C electrode after 100 electrochemical cycles by TEM, as shown in Fig. S3.† It is clear that the Cu6Sn5 nanoparticles do not aggregate after cycling and they are still uniformly and firmly anchored on the surface of carbon nanosheets, which is almost the same as the morphology of the pristine product (Fig. 3b) except for the covered SEI layer. This demonstrates a strong interaction between Cu6Sn5 nanoparticles and carbon nanosheets as well as the superior mechanical flexibility of 3D porous carbon nanosheet networks, which can restrain the aggregation of Cu6Sn5 nanoparticles and the cracking of the electrode during charge/discharge cycling. As a result, the 3D PCNNWs-Cu6Sn5@C electrode displays an excellent cycling stability.
The 3D PCNNWs-Cu6Sn5@C anode not only presents a stable cycle performance, but also exhibits an excellent rate capability, as illustrated in Fig. 4c and d and Table 1S.† The reversible capacities at 0.2, 0.5, 1, 2, 5, and 10 A g−1 are 523, 443, 395, 327, 281, and 203 mA h g−1, respectively. When compared with the as-prepared Cu6Sn5/carbon composite (Fig. 4c) and some available representative results of the Cu–Sn alloy anodes reported by other researchers (Table S1†), notably, for the 3D PCNNWs-Cu6Sn5@C, the achieved rate capability is superior to the Cu6Sn5/carbon composite prepared without NaCl template and recent reported Cu–Sn alloy synthesized by complex methods.32–37,40,41 This good rate capability may benefit from the combination of the nano-Cu6Sn5@carbon with short path for Li+ diffusion and 3D carbon nanosheet networks with high electric conductivity.
In order to gain further understanding of the excellent rate performance for the 3D PCNNWs-Cu6Sn5@C, the coin cells of 3D PCNNWs-Cu6Sn5@C and Cu6Sn5/carbon composite after the first cycle were measured by electrochemical impedance spectroscopy (EIS). Fig. 4f shows the Nyquist plots, which are composed of a depressed semicircle in the high-medium frequency region and an oblique straight line in the low frequency region. According to the previous reports,13,14 the depressed semicircle relates to the charge transfer resistances (Rct) and the oblique straight line corresponds to Warburg impedance (ZW) associated with the Li+ diffusion in the bulk of anode material. The 3D PCNNWs-Cu6Sn5@C electrode presents a smaller high-medium frequency semicircle compared with Cu6Sn5/carbon composite, indicating that a good electronic conductivity can be achieved owing to the 3D porous carbon nanosheet networks anchored with nano-sized Cu6Sn5@carbon, which results in a superior rate capability, as discussed above (Fig. 4c and d). To sum up, the superior electrochemical performance can be ascribed to the unique structure of 3D PCNNWs-Cu6Sn5@C that offers porous structure for electrolyte infiltration, higher surface area for more active sites, copper in Cu6Sn5 for relaxing the large volume, nano-sized alloy for shorter path of Li+ diffusion, carbon shells outside Cu6Sn5 for minimizing the agglomeration, 3D conducting carbon matrix for fast electron transport and more freedom space for volume change, which can keep a stable connection between active material and the current collector and enhance the reaction kinetics.
Conclusions
In summary, we have developed a practical synthetic method to prepare 3D porous carbon nanosheet networks anchored with Cu6Sn5@carbon nanoparticles (10–35 nm) that exhibiting an outstanding electrochemical performance for lithium ion battery anodes. Importantly, compared to the previous work, this strategy is easier to tailor and control the component of Cu–Sn alloy, nanoparticle size, uniform distribution, and porous conductive network structure. As a result, such a nanostructured composite exhibits a high reversible capacity (523 mA h g−1 at 0.2 A g−1), excellent rate capability (443, 395, 327, 281, and 203 mA h g−1 at 0.5, 1, 2, 5, and 10 A g−1, respectively) and superior cycling stability (capacity retention attained 92.3% after cycled at 1 A g−1 for 200 times), which could maximum of electrochemical activity of Cu6Sn5 and carbon nanosheet networks and highlight the preeminence of the anchoring of Cu6Sn5 nanoparticles on 3D porous carbon nanosheet networks. Thus it is a promising anode material for the next generation LIBs with high energy and power density.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants No. 51404055; No. 51374056; No. 51571054), the Special Fund for Basic Scientific Research of Central Colleges, Northeastern University (No. N142304002; N100123003; N120523001), the Natural Science Foundation of Hebei (No. B2015501020; No. E2013501135), the Youth Foundation of Hebei Educational Committee (No. QN2014325), the program for New Century Excellent Talents in University (No. NCET-10-0304), the Key Technologies R & D Program of Qinhuangdao of Hebei Province (No. 201402B005), and the Doctoral Scientific Research Foundation of Northeastern University at Qinhuangdao, China (No. XNB201511).References
- W. J. Zhang, J. Power Sources, 2011, 196, 13–24 CrossRef CAS
.
- C. M. Park, J. H. Kim, H. Kim and H. J. Sohn, Chem. Soc. Rev., 2010, 39, 3115–3141 RSC
.
- X. L. Wang, W. Q. Han, J. Chen and J. Graetz, ACS Appl. Mater. Interfaces, 2010, 2, 1548–1551 CAS
.
- J. Wolfenstine, S. Campos, D. Foster, J. Read and W. K. Behl, J. Power Sources, 2002, 109, 230–233 CrossRef CAS
.
- M. G. Kim, S. Sim and J. Cho, Adv. Mater., 2010, 22, 5154–5158 CrossRef CAS PubMed
.
- N.-S. Choi, Y. Yao, Y. Cui and J. Cho, J. Mater. Chem., 2011, 21, 9825–9840 RSC
.
- M. Tian, W. Wang, Y. J. Wei and R. G. Yang, J. Power Sources, 2012, 211, 46–51 CrossRef CAS
.
- J.-G. Kang, G.-H. Lee, K.-S. Park, S.-O. Kim, S. J. Lee, D.-W. Kim and J.-G. Park, J. Mater. Chem., 2012, 22, 9330–9337 RSC
.
- B. Luo, Y. Fang, B. Wang, J. H. Zhou, H. H. Song and L. J. Zhi, Energy Environ. Sci., 2012, 5, 5226–5230 CAS
.
- Y. Yu, C. H. Chen and Y. Shi, Adv. Mater., 2007, 19, 993–997 CrossRef CAS
.
- X. F. Li, A. Dhanabalan, L. Gu and C. L. Wang, Adv. Energy Mater., 2012, 2, 238–244 CrossRef CAS
.
- G. Derrien, J. Hassoun, S. Panero and B. Scrosati, Adv. Mater., 2007, 19, 2336–2340 CrossRef CAS
.
- Z. Q. Zhu, S. W. Wang, J. Du, Q. Jin, T. R. Zhang, F. Y. Cheng and J. Chen, Nano Lett., 2014, 14, 153–157 CrossRef CAS PubMed
.
- J. Qin, C. N. He, N. Q. Zhao, Z. Y. Wang, C. S. Shi, E. Z. Liu and J. J. Li, ACS Nano, 2014, 8, 1728–1738 CrossRef CAS PubMed
.
- N. Zhang, Q. Zhao, X. P. Han, J. G. Yang and J. Chen, Nanoscale, 2014, 6, 2827–2832 RSC
.
- Y. H. Xu, Q. Liu, Y. J. Zhu, Y. H. Liu, A. Langrock, M. R. Zachariah and C. S. Wang, Nano Lett., 2013, 13, 470–474 CrossRef CAS PubMed
.
- Y. Zhang, L. Jiang and C. Wang, Nanoscale, 2015, 7, 11940–11944 RSC
.
- J.-M. Tarascon and M. Armand, Nature, 2001, 414, 359–364 CrossRef CAS PubMed
.
- Y. Kim, Solid State Ionics, 2003, 160, 235–240 CrossRef CAS
.
- K. K. D. Ehinon, S. Naille, R. Dedryvère, P.-E. Lippens, J.-C. Jumas and D. Gonbeau, Chem. Mater., 2008, 20, 5388–5398 CrossRef CAS
.
- J.-T. Li, J. Swiatowska, V. Maurice, A. Seyeux, L. Huang, S.-G. Sun and P. Marcus, J. Phys. Chem. C, 2011, 115, 7012–7018 CAS
.
- L. Shi, H. Li, Z. Wang, X. Huang and L. Chen, J. Mater. Chem., 2001, 11, 1502–1505 RSC
.
- H. Mukaibo, T. Osaka, P. Reale, S. Panero, B. Scrosati and M. Wachtler, J. Power Sources, 2004, 132, 225–228 CrossRef CAS
.
- S. H. Lee, M. Mathews, H. Toghiani, D. O. Wipf and C. U. Pittman, Chem. Mater., 2009, 21, 2306–2314 CrossRef CAS
.
- X. L. Wang, M. Feygenson, H. Y. Chen, C. H. Lin, W. Ku, J. M. Bai, M. C. Aronson, T. A. Tyson and W. Q. Han, J. Am. Chem. Soc., 2011, 133, 11213–11219 CrossRef CAS PubMed
.
- M. Chamas, P.-E. Lippens, J.-C. Jumas, K. Boukerma, R. Dedryvère, D. Gonbeau, J. Hassoun, S. Panero and B. Scrosati, J. Power Sources, 2011, 196, 7011–7015 CrossRef CAS
.
- C.-j. Liu, F.-h. Xue, H. Huang, X.-h. Yu, C.-j. Xie, M.-s. Shi, G.-z. Cao, Y.-g. Jung and X.-l. Dong, Electrochim. Acta, 2014, 129, 93–99 CrossRef CAS
.
- B. Feng, J. Xie, G.-S. Cao, T.-J. Zhu and X.-B. Zhao, New J. Chem., 2013, 37, 474–480 RSC
.
- Y. Gu, F. Wu and Y. Wang, Adv. Funct. Mater., 2013, 23, 893–899 CrossRef CAS
.
- N. Mahmood, C. Z. Zhang, F. Liu, J. H. Zhu and Y. L. Hou, ACS Nano, 2013, 7, 10307–10318 CrossRef CAS PubMed
.
- W. Choi, J. Y. Lee and H. S. Lim, Electrochem. Commun., 2004, 6, 816–820 CrossRef CAS
.
- H. C. Shin and M. Liu, Adv. Funct. Mater., 2005, 15, 582–586 CrossRef CAS
.
- W. J. Cui, F. Wang, J. Wang, H. J. Liu, C. X. Wang and Y. Y. Xia, J. Power Sources, 2011, 196, 3633–3639 CrossRef CAS
.
- W. X. Lei, Y. Pan, Y. C. Zhou, W. Zhou, M. L. Peng and Z. S. Ma, RSC Adv., 2014, 4, 3233–3237 RSC
.
- D. B. Polat, J. Lu, A. Abouimrane, O. Keles and K. Amine, ACS Appl. Mater. Interfaces, 2014, 6, 10877–10885 CAS
.
- F. Wang, J. Yi, Y. G. Wang and Y. Y. Xia, Nanotechnology, 2013, 24, 424010–424014 CrossRef PubMed
.
- J. Z. Chen, L. Yang, S. H. Fang, Z. X. Zhang and S. I. Hirano, Electrochim. Acta, 2013, 105, 629–634 CrossRef CAS
.
- S. H. Lee, S. H. Yu, J. E. Lee, A. H. Jin, D. J. Lee, N. Lee, H. Jo, K. Shin, T. Y. Ahn, Y. W. Kim, H. Choe, Y. E. Sung and T. Hyeon, Nano Lett., 2013, 13, 4249–4256 CrossRef CAS PubMed
.
- H. P. Xu, S. Yuan, Z. Y. Wang, Y. Zhao, J. H. Fang and L. Y. Shi, RSC Adv., 2014, 4, 8472–8480 RSC
.
- Q. G. Han, Z. Yi, Y. Cheng, Y. M. Wu and L. M. Wang, RSC Adv., 2016, 6, 15279–15285 RSC
.
- X. Y. Fan, Y. X. Shi, Y. Cui, D. L. Li and L. Gou, Ionics, 2015, 21, 1909–1917 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The Cu–Sn phase diagram, TG–DTA curves of the 3D PCNNWs-Cu6Sn5@C composite in air, TEM images of 3D PCNNWs-Cu6Sn5@C electrode after 100 cycles with different magnification, and electrochemical performances of 3D PCNNWs-Cu6Sn5@C and some representative Cu–Sn alloy anodes in literature. See DOI: 10.1039/c6ra04778e |
| This journal is © The Royal Society of Chemistry 2016 |