Dy3+ doped thermally stable garnet-based phosphors: luminescence improvement by changing the host-lattice composition and co-doping Bi3+
Abstract
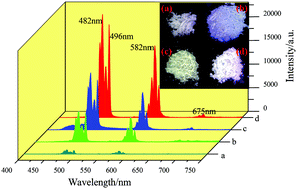
A series of (Gd1−xYx)2.94−yAl5O12: 0.06Dy3+,yBi3+(x = 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, y = 0.000, 0.015, 0.030, 0.045, 0.060) phosphors has been prepared by a sol–gel combustion method. X-ray diffraction (XRD), scanning electron microscopy (SEM), photoluminescence (PL), diffuse reflection, fluorescence decay curves, temperature-dependent photoluminescence and CIE color coordinates were used to characterize the prepared phosphors. The emission intensity of Dy3+ was greatly improved by co-doping the sensitizer of Bi3+ ions and changing the composition of the matrix via replacing gadolinium with Y3+ ions. The influence of different Y3+ and Bi3+ concentrations on the properties of the phosphor were discussed. The optimized composition was determined to be (Gd0.2Y0.8)2.835Al5O12: 0.06Dy3+,0.045Bi3+. Its intensity is almost double the intensity in YAG: Dy3+,Bi3+, four times stronger than that of GAG: Dy3+,Bi3+ and 15 times stronger than that of YGAG: Dy3+. The band gap and decay curves of the phosphors were investigated to explore the relationship between the intensity and the content of Y3+. In addition, good thermal stability of (Gd0.2Y0.8)2.835Al5O12: 0.06Dy3+,0.045Bi3+ was observed according to the temperature-dependent PL spectra. The enhanced luminescence intensity and good thermal stability of the phosphor are conducive to improved application in the field of white light-emitting diodes (W-LEDs).

 Please wait while we load your content...
Please wait while we load your content...