A colorimetric and ratiometric fluorescence sensor for sensitive detection of fluoride ions in water and toothpaste†
Abstract
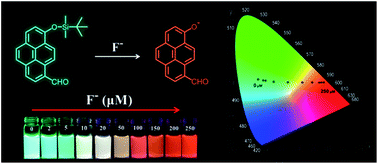
Fluoride is a well-known anion that plays a significant physiological role. Sensitive and quantitative sensing of fluoride is of great importance to public health investigation. A colorimetric and ratiometric fluorescence sensor for fluoride ions based on silyl capped hydroxylpyrenealdehyde is designed and synthesized. The sensor detects fluoride ions through the desilylation mediated by fluoride ions and the consequent spectral change of the pyrene derivative. A significant absorption change from 420 to 523 nm in the visual region and a fluorescence shift from 492 to 603 nm can be observed upon addition of fluoride ions of tens of ppb, enabling direct observation with the bare eye. Based on the ratiometric fluorescence with up to 255-fold enhancement, the sensor can rapidly and selectively detect F− in water with a limit as low as 2.7 ppb, and furthermore, the sensor is successfully applied for determining the levels of F− in commercially available toothpaste.

 Please wait while we load your content...
Please wait while we load your content...