Controlled synthesis of an enzyme–inorganic crystal composite assembled into a 3D structure with ultrahigh enzymatic activity†
Xiufu Huaab,
Yi Xing*a and
Xuan Zhangc
aDepartment of Environmental Engineering, University of Science and Technology Beijing, 100083, China. E-mail: xingyi@ustb.edu.cn
bDepartment of Scientific Research and Development, Tsinghua University, Beijing, 100084, China
cDepartment of Chemical Engineering, Northwest University, Xi’an, 710069, China
First published on 22nd April 2016
Abstract
In this study, we report a method for the controlled synthesis of an enzyme–inorganic crystal composite assembled into a 3D structure with flower-like shape, which shows an ultrahigh enzymatic activity. Using ethylenediaminetetraacetic acid (EDTA) as the chelating compound, the addition of a protein aqueous solution in phosphate saline buffer into a water solution containing Cu(II) resulted in a 3D architecture made of protein and flower-like shaped copper phosphate crystals with controllable size ranging from approximately 1 μm to 15 μm. The incorporated laccase showed ultrahigh activity, which was about 7–11 times higher compared to free laccase in solution. The immobilized laccase–copper phosphate hybrid microflowers can be applied for rapid and sensitive detection of phenol in water. Repeated use of immobilized laccase microflowers was demonstrated for 60 cycles during a period of two months.
The self-assembly of biomolecules with inorganic material building blocks into 1D, 2D and even complex 3D architectures has attracted a lot of research interest due to its appealing application in bio-nanotechnology, biomaterials, biocatalysis, and biosensing.1,2 Recently, Ge et al. and other groups reported a novel method of preparing various types of self-assembled protein–inorganic crystal composites with flower-like shapes,3–6 which have much higher enzymatic activities than both free enzymes and traditional immobilized enzymes.7,8 These novel types of immobilized enzymes have been recognized as very promising for wide applications in diverse fields.9 While the discovery of these new types of enzyme–inorganic hybrid microflowers is certainly important, the strategy of synthesizing the 3D enzyme–inorganic crystal composites in a controlled manner has received almost no attention. In addition, in the above-mentioned studies, enzyme–inorganic hybrid microflowers with average sizes of over 10 μm were prepared. We propose that by further decreasing the overall size of the 3D structure, the activity of the incorporated enzyme might be increased due to the reduced mass transfer limitation for enzymatic catalysis.10–12 However, the previously reported method of synthesizing enzyme–inorganic hybrid microflowers in aqueous solution could not obtain composites with relatively small sizes.3–6 Therefore, to uncover the size–activity relationship of the 3D enzyme–inorganic crystal composite with flower-like shape, an effective preparation method with controllable sizes of the product is urgently required.
The present study described a simple method to fabricate 3D flower-like laccase–copper phosphate composites with controllable size in the presence of a commonly used copper ion chelating compound (EDTA). As shown in Fig. 1, CuSO4 water solution containing EDTA was added to phosphate buffered saline (PBS) containing laccase, and incubated for three days to completely form the laccase–inorganic hybrid microflowers. The relationship between the size of the enzyme microflowers and the enzymatic activity was studied. The laccase–copper phosphate microflowers with the highest enzymatic activity were then physically immobilized on a commercial disposable syringe filter with a cellulose acetate membrane (pore size of 0.2 μm). This allowed a rapid analysis of phenol compounds in aqueous solution which passed through the laccase microflower incorporated membrane, on the basis of laccase-catalyzed oxidative coupling of phenol with 4-aminoantipyrine to form antipyrine dyes.13 In addition, the laccase microflower incorporated membrane can be reused for many times with unchanged enzymatic activity after washing the membrane with water.
For the preparation of the enzyme–copper phosphate microflowers, in a typical experiment, 0.8 mM CuSO4 water solution was added to phosphate buffered saline (PBS) containing 0.1 g L−1 laccase at a pH of 7.4 and 25 °C. After a three-day incubation period at 25 °C, a precipitate of laccase microflowers appeared with porous, flower-like structures as shown in the SEM image in Fig. 2(a). The as-prepared laccase–copper phosphate microflowers without EDTA have typical sizes around 15 μm, which agrees well with the previous study.3 With the addition of EDTA to the solution, the size of the obtained laccase microflowers can be easily controlled. As shown in Fig. 2(b–f), at an EDTA concentration range of 10–50 mM in the synthesis solution, the size of the obtained laccase–copper phosphate microflowers varied from around 6 μm to 1 μm.
 | ||
| Fig. 2 SEM images of the laccase–copper phosphate hybrid microflowers prepared with different concentrations of EDTA, (a) 0 mM, (b) 10 mM, (c) 20 mM, (d) 30 mM, (e) 40 mM, and (f) 50 mM. | ||
The morphology and high-resolution image of the laccase microflowers prepared without EDTA are given in Fig. 3(a and b), which clearly show the flower-shaped nanostructures. Calcination of the microflowers at 350 °C was used to investigate the location of protein in the microflowers, and resulted in a loss of the flower structure (Fig. 3(c)), suggesting that the protein was mainly present in the core of the microflower and served as the ‘glue’ to bind the petals together. This result agreed well with the finding of the previous study.3 The X-ray diffraction pattern (XRD) of the microflower powder (Fig. 3(d)) suggested that the inorganic crystal formed in the microflowers was Cu3(PO4)2·3H2O (JPSCD 00-022-0548). As discussed by Ge et al., aggregates of protein and copper ions were first formed due to the coordination reaction between protein and Cu(II) which provide sites for the nucleation of copper phosphate crystals.3 The flower-like copper phosphate crystals then gradually grow from these nucleation sites to eventually form the enzyme–inorganic hybrid microflowers.
Fig. 4(a) gives the high-resolution SEM image of the petal of the microflower, which clearly shows that the petal was made of self-assembled crystals. The crystals were possibly bound together with protein molecules to form the petal of the microflower. Fig. 4(b) shows the high-resolution TEM image of the microflower, demonstrating the dense core packed with protein and copper phosphate crystals and the light shell of relatively loose petals made of copper phosphate crystals. On the shell of the microflower which consists of the petals, a clear crystal lattice structure was observed as shown in Fig. 4(b) (inset).
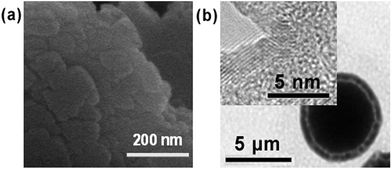 | ||
| Fig. 4 (a) HR-SEM image of the self-assembled crystals on a petal of a microflower. (b) HR-TEM image of a microflower, inset showing the lattice of the crystal. | ||
In our study, by using EDTA as the chelating compound for Cu(II), a sustained release of Cu(II) occurred during the nucleation and crystal growth process. A higher EDTA concentration resulted in a slower release of Cu(II) from the Cu–EDTA complex and allowed a more complete nucleation with more crystal growth sites. Therefore, with the constant input of starting materials and at high concentrations of EDTA, the size of the microflowers became smaller due to more microflowers being formed. The relationship between the size of the microflowers and EDTA concentration is presented in Table 1. By measuring the concentration of unencapsulated protein in solution, the encapsulation yield and weight percentage of laccase in the microflowers in the presence of different EDTA concentrations were calculated and are presented in Table 1.
| Sample | EDTA concentration (mM) | Encapsulation ratio | Enzyme loading (w/w) | Average size (μm) |
|---|---|---|---|---|
| 1 | 0 | 85% | 8% | 15 |
| 2 | 10 | 90% | 8% | 6 |
| 3 | 20 | 92% | 9% | 4 |
| 4 | 30 | 95% | 8% | 2 |
| 5 | 40 | 97% | 10% | 2 |
| 6 | 50 | 97% | 11% | 1 |
The enzymatic activity of laccase incorporated into the microflowers was measured by the standard method using 2,6-dimethoxyphenol (DMP) as the substrate at the same protein content.14 As shown in Fig. 5(a), at optimal pH (the influence of pH on enzyme activity is presented in ESI†) all of the as-prepared laccase microflowers have increased activity compared to free laccase in aqueous solution. As proposed by the previous study, the increased laccase activity in microflowers was due to the cooperative interaction between laccase and copper ions in crystals and the suitable microenvironment created by the microflowers.3 In our study, we found as expected that a smaller size of the microflowers resulted in a higher enzymatic activity. This is reasonable because laccase was encapsulated inside the microflowers; a smaller size of the microflowers would generate a higher surface–volume ratio of the nanobiocatalyst and reduce the mass transport resistance for the enzymatic reaction catalyzed by laccase located inside the microflowers. As shown in Fig. 5(a), the highest enzymatic activity of 1100% compared to free laccase in solution was achieved when the size of the microflowers was reduced to 1 μm using 50 mM EDTA. The stability of the laccase microflowers with a size of 1 μm was determined by incubating in phosphate buffer solution (50 mM, pH 7.0) at room temperature. As shown in Fig. 5(b), the laccase microflowers preserved most of the activity during a period of two weeks while free laccase lost over 80% of its activity.
As a demonstration to show the application of the laccase microflowers, a water suspension containing laccase microflowers with the highest enzymatic activity (2 g L−1 microflowers, 0.2 g L−1 protein, 0.3 mL) was injected into a commercial syringe filter with cellulose acetate membrane (pore size: 0.2 μm). This allows physical immobilization of the microflowers on the membrane surface. Because the size of the microflowers is larger than the pore size of the membrane, the microflowers can be easily immobilized on the membrane. No obvious presence of enzyme microflowers was detected in the filtered solution. Free laccase was also injected into the filter by the same procedure for comparison with the laccase microflowers. For the detection of phenol in water using the laccase microflowers, in a typical experiment, 2 mL of phenol solution in 50 mM pH 6.0 phosphate buffer was first mixed with 2 mL of 4-aminoantipyrine water solution (1 g L−1). Then, 400 μL of the mixture was injected into the membrane filter and incubated at room temperature for 5 minutes. The laccase microflowers catalyzed the fast oxidative coupling of phenol with 4-aminoantipyrine to form an antipyrine-dye, which can be detected by a UV-Vis spectrophotometer at 495 nm.13 After incubation, the solution was pushed out by a syringe and collected for measurement in the UV-Vis spectrophotometer. Fig. 5(c) shows analysis of the samples with different concentrations (from 0.5 to 100 μg mL−1 in 50 mM pH 6.0 phosphate buffer). A linear relationship between the absorption intensity at 495 nm and the concentration of phenol was established as shown in Fig. 5(c).
Analysis of a water solution containing 100 μg mL−1 of phenol was done to test the reusability of the laccase microflower in the filter. After each run, the filter was washed with 2 mL of DI water, dried at room temperature, and then subjected to the next run. The test of reusability was conducted once a day for two months. Fig. 5(d) shows the excellent stability of the laccase microflower incorporated membrane filter which was reused for 60 cycles in two months with more than 80% relative activity retained. The highly preserved activity of the laccase microflowers on the membrane at room temperature agrees well with the high stability of laccase microflowers in aqueous solution. Phenols in water are toxic to aquatic organisms. Gas chromatography (GC) and liquid chromatography (LC) have been conventionally used to determine the concentration of phenolic compounds. However, these methods usually require time-consuming procedures and highly trained technicians, and are much more suitable for accurate analysis in the lab. Many great efforts have been made to achieve facile and fast detection of phenol in water.15–17 This study reported a facile method that can detect phenol in water from 0.5 to 50 μg mL−1, meeting the requirements of in-the-field analysis of phenols in environmental water. The major advantages of this method are the convenience of fast detection and the enzymatic reaction that is specific to phenolic compounds.
Conclusions
In summary, we reported a new method for the controlled synthesis of laccase–copper phosphate microflowers with tunable size by adding EDTA. The sizes of the laccase microflowers ranged from 15 μm to 1 μm with a concentration of EDTA from 0 mM to 50 mM in solution. With the size of the laccase microflowers reduced, the activity of the laccase microflowers was increased from ∼700% to ∼1100%, compared to that of free laccase in solution. The immobilization of laccase microflowers on a membrane was readily achieved by physical adsorption. The immobilized laccase microflowers can be used for the rapid detection of phenols in water solution. Moreover, the highly concentrated laccase on the membrane provides a concentrated signalling interface and thus improves the sensitivity of the detection. The high stability of laccase in the microflowers, in comparison to its free form, ensures high reproducibility of the detection.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51104009), Beijing Nova Program (No. Z111106054511043), Beijing Outstanding Talent Nurture and Funding Scheme (No. 2012D009006000003), the Fundamental Research Funds for the Central Universities (No. FRF-TP-12-011B), The Special Fund of State Key Joint Laboratory of Environment Simulation and Pollution Control (No. 15K03ESPCT).Notes and references
- A. J. Patil, M. Lia and S. Mann, Nanoscale, 2013, 5, 7161 RSC.
- S. Pal, Z. T. Deng, B. Q. Ding, H. Yan and Y. Liu, Angew. Chem., Int. Ed., 2010, 49, 2700 CrossRef CAS PubMed.
- J. Ge, J. D. Lei and R. N. Zare, Nat. Nanotechnol., 2012, 7, 428 CrossRef CAS PubMed.
- L. B. Wang, Y. C. Wang, R. He, A. Zhuang, X. Wang, J. Zeng and J. G. Hou, J. Am. Chem. Soc., 2013, 135, 1272 CrossRef CAS PubMed.
- Z. Lin, Y. Xiao, Y. Q. Yin, W. L. Hu, W. Liu and H. H. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 10775 CAS.
- X. L. Wang, J. F. Shi, Z. Li, S. H. Zhang, H. Wu, Z. Y. Jiang, C. Yang and C. Y. Tian, ACS Appl. Mater. Interfaces, 2014, 6, 14522 CAS.
- J. Kim and J. W. Grate, Nano Lett., 2003, 3, 1219 Search PubMed.
- H. R. Luckarift, J. C. Spain, R. R. Naik and M. O. Stone, Nat. Biotechnol., 2004, 22, 211 CrossRef CAS PubMed.
- X. Wu, M. Hou and J. Ge, Catal. Sci. Technol., 2015, 5, 5077 CAS.
- J. Ge, D. N. Lu, Z. Z. Li and Z. Liu, Biochem. Eng. J., 2009, 44, 53 CrossRef CAS.
- J. Ge, C. Yang, J. Zhu, D. N. Lu and Z. Liu, Top. Catal., 2012, 55, 1070 CrossRef CAS.
- R. Wang, Y. Zhang, D. N. Lu, J. Ge, Z. Liu and R. N. Zare, WIREs Nanomedicine and Nanobiotechnology, 2013, 5, 320 CrossRef CAS PubMed.
- E. Morita and E. Nakamura, Anal. Sci., 2011, 27, 489 CrossRef CAS PubMed.
- X. L. Zhang, M. Hua, L. Lv and B. C. Pan, Sci. Rep., 2015, 5, 8253 CrossRef CAS PubMed.
- J. Singh, P. Kalita, M. K. Singh and B. D. Malhotra, Appl. Phys. Lett., 2011, 98, 123702 CrossRef.
- J. Singh, A. Roychoudhury, M. Srivastava, V. Chaudhary, R. Prasanna, D. W. Lee, S. H. Lee and B. D. Malhotra, J. Phys. Chem. C, 2013, 117, 8491 CAS.
- A. K. Das, R. K. Layek, N. H. Kim, D. Jung and J. H. Lee, Nanoscale, 2014, 6, 10657 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04664a |
| This journal is © The Royal Society of Chemistry 2016 |



