Photochemical reactions of g-C3N4-based heterostructured composites in Rhodamine B degradation under visible light
Yumeng Liu,
Junpeng Wang and
Ping Yang*
School of Material Science and Engineering, University of Jinan, Jinan, 250022, P. R. China. E-mail: mse_yangp@ujn.edu.cn; Fax: +86 531 87974453; Tel: +86 531 89736225
First published on 30th March 2016
Abstract
Highly efficient visible-light-driven Ag2O/g-C3N4, Ag/g-C3N4, and BiOBr/g-C3N4 heterostructured photocatalysts were prepared by solution synthesis methods at room temperature. Compared with Ag/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mass ratio) and Ag2O/g-C3N4 (2
30 mass ratio) and Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio) photocatalysts, the BiOBr/g-C3N4 composite (1
1 mass ratio) photocatalysts, the BiOBr/g-C3N4 composite (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) displayed enhanced photocatalytic activities for Rhodamine B (RhB) degradation under visible-light irradiation. The reaction kinetics of phenol RhB dye photodegradation of Ag2O/g-C3N4 (1
3) displayed enhanced photocatalytic activities for Rhodamine B (RhB) degradation under visible-light irradiation. The reaction kinetics of phenol RhB dye photodegradation of Ag2O/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratios) and BiOBr/g-C3N4 were fitted with the pseudo-first-order model, ln(C0/C) = kt, while the data of Ag/g-C3N4 and pure g-C3N4, Ag2O/g-C3N4 (1
1 mass ratios) and BiOBr/g-C3N4 were fitted with the pseudo-first-order model, ln(C0/C) = kt, while the data of Ag/g-C3N4 and pure g-C3N4, Ag2O/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mass ratio) were fitted with the zero-order model. The former has enhanced photocatalytic activity, and BiOBr/g-C3N4 composites exhibit the highest degrading rate for RhB. The enhanced photocatalytic activity of two dimensional BiOBr/g-C3N4 photocatalysts was mainly attributed to the formation of type II p–n heterojunctions, as well as the well-matched band gap and the synergetic effects between two components, which accelerate the separation efficiency of photogenerated electrons–holes at the interface. Finally, possible photocatalytic and charge separation mechanisms of Ag2O/g-C3N4 and BiOBr/g-C3N4 composites were proposed via active species capture experiments.
20 mass ratio) were fitted with the zero-order model. The former has enhanced photocatalytic activity, and BiOBr/g-C3N4 composites exhibit the highest degrading rate for RhB. The enhanced photocatalytic activity of two dimensional BiOBr/g-C3N4 photocatalysts was mainly attributed to the formation of type II p–n heterojunctions, as well as the well-matched band gap and the synergetic effects between two components, which accelerate the separation efficiency of photogenerated electrons–holes at the interface. Finally, possible photocatalytic and charge separation mechanisms of Ag2O/g-C3N4 and BiOBr/g-C3N4 composites were proposed via active species capture experiments.
Introduction
Semiconductor photocatalysis has emerged as one of the most promising technologies for environmental remediation and solar energy conversion.1 The growing concerns about visible-light-driven photocatalysts have stimulated extensive studies. Of the well-known semiconductor photocatalysts, TiO2 has attracted more attention owing to its stability, nontoxicity, and low-cost.2 However, because of relatively wide band gap and fast recombination of the photo-generated electron–hole pairs, the enhancement of the visible-light photocatalytic activity is still very limited.3 Therefore, it is still a great challenge and highly crucial to explore novel photocatalytic materials with visible light response wavelength range.Graphitic carbon nitride (g-C3N4), a polymeric metal-free semiconductor has been recently focused on the visible-light photocatalytic field with the advantages of good stability and an appealing electronic structure with a medium band gap of 2.7 eV,4 which fulfills the basic requirements as a photocatalyst for water splitting and/or organic pollutant decomposition. g-C3N4 can easily be synthesized via direct polymerization of nitrogen-containing such as cyanamide, dicyandiamide, melamine, urea, and thiourea. Nevertheless, the low quantum efficiency, and the fast recombination of photogenerated electron–hole pairs, the lack of absorption above 460 nm may still restrict the photocatalytic activities of pure g-C3N4.5 In order to overcome these problems and improve the photochemical reactivity of g-C3N4, many strategies have been investigated to improve the photocatalytic activity of g-C3N4, such as transition metal doping, noble metals modification, and heterostructured nanocomposites.6,14 Heterostructured composites have become one of the hottest research topics. Various g-C3N4 based hybrid photocatalysts including g-C3N4/Bi2WO6,6 g-C3N4/Co3O4,7 g-C3N4/Fe2O3,8 and g-C3N4/NiS9 have been developed to further extend the visible light absorption range and photogenerated carrier separation efficiency. Recently, Ag-based photocatalysts attracts growing attention which can be used to combine with g-C3N4 to promote the separation of photogenerated carriers and improve their photocatalytic activities. Xu et al. prepared AgX/g-C3N4 (X = Br and I) composites with high photocatalytic activities in methyl orange (MO) degradation.10 The preparation of a series of ternary Ag/Ag3PO4/g-C3N4 hybrid photocatalysts, which display enhanced photocatalytic activity, was reported.11 These studies clearly indicated the enhanced photocatalytic activities by incorporating Ag-based materials with g-C3N4, and showed superior photocatalytic activities to degrade pollutants under visible-light irradiation. However, AgX and Ag3PO4 photocatalysts are unstable under light illumination, thus, it is hard to precise control the mass ratios between the components. Ag2O, as a p-type semiconductor with a narrow band gap of 1.46 eV, has been found to be a self-stable and highly efficient photocatalyst under visible light.12 It has been widely used as an efficient sensitizer to tune the light response of wide-band-gap semiconductors into the visible region and improve their photocatalytic activities, such as Ag2O/TiO2.13 The combination of g-C3N4 and Ag2O that possesses well matched band structure can easily fabricate a p–n heterojunction, which will bring more effective interface transfer of photogenerated electrons and holes in comparison with pure Ag2O and g-C3N4. Moreover, semiconductor photocatalysts modified by pure noble metal nanoparticles could also exhibit enhanced photocatalytic activities, such as Au/g-C3N4.14 Therefore, it is of vital importance to explore Ag-based photocatalysts with visible-light response. As we all known, the studies on the Ag2O/g-C3N4 system are plentiful, but no study has compared Ag2O/g-C3N4 with Ag/g-C3N4 under visible light.
Bismuth oxyhalides (BiOX, X = Cl, Br, I), new candidates of prospective photocatalysts, have exhibited enhanced photocatalytic activities in degrading organic dyes compared to common photocatalyst such as TiO2 and ZnO as a result of their intrinsic lamellar structures.15 Although pure BiOBr materials arouse great interest for its visible light response and high stability, as well as narrow band gap, it is still necessary to construct heterostructured materials to further improve its photocatalytic activity for practical applications as a result of its rapid combination of photogenerated charge carriers of pure BiOBr. Visible-light-responsive BiOBr–ZnFe2O4 hetero-junction photocatalysts were prepared by a precipitation deposition method which exhibited better photocatalytic activity compared with pure BiOBr for the degradation of Rhodamine B (RhB).16 Moreover, both BiOBr/BiWO3 (ref. 17) and BiOBr/Bi2O3 (ref. 18) composites showed enhanced photocatalytic activities. Due to well matched band structure, the combination of g-C3N4 and BiOBr can be easily fabricated into two dimensional (2D) p–n heterojunction composites, which will bring more effective separation and transfer of photogenerated charges.
Herein, highly efficient visible-light-driven heterostructured photocatalysts including Ag/g-C3N4, Ag2O/g-C3N4, and BiOBr/g-C3N4 were prepared by solution reactions at room temperature, which could be quantitatively found out the optimal photocatalysts. Ag2O/g-C3N4 and BiOBr/g-C3N4 with p–n heterostructured photocatalysts revealed high photo-degradation efficiency for RhB compared with Ag/g-C3N4, all of which possessed ideal photocatalytic activity and stability under visible light irradiation compared with the pure g-C3N4. The reaction kinetics of phenol RhB dye photodegradation of Ag2O/g-C3N4 with mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and BiOBr/g-C3N4 composites were fitted with the pseudo-first-order model, ln(C0/C) = kt, while the experimental data of Ag/g-C3N4, Ag2O/g-C3N4 with a mass ratio of 1
1 and BiOBr/g-C3N4 composites were fitted with the pseudo-first-order model, ln(C0/C) = kt, while the experimental data of Ag/g-C3N4, Ag2O/g-C3N4 with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and pure g-C3N4 were fitted with the zero-order kinetics model. We then conclude that the as-prepared samples which meet the pseudo-first-order model are optimal photocatalysts. Meanwhile, we also proposed possible mechanisms for the enhanced activity of Ag2O/g-C3N4, BiOBr/g-C3N4 composites on account of the experimental results.
20 and pure g-C3N4 were fitted with the zero-order kinetics model. We then conclude that the as-prepared samples which meet the pseudo-first-order model are optimal photocatalysts. Meanwhile, we also proposed possible mechanisms for the enhanced activity of Ag2O/g-C3N4, BiOBr/g-C3N4 composites on account of the experimental results.
Experimental
Chemicals
Melamine (C3H6N6), p-benzoquinone (PBQ), triethanolamine (TEA), methanol, silver nitrate (AgNO3), potassium bromide (KBr) and sodium hydroxide (NaOH) were obtained from Sinopharm Chemical Reagent Co., Ltd, China. Bismuth nitrate was purchased from Kermal, Tianjin. All chemicals were used as received without further purification, and all aqueous solutions were prepared with ultrapure water (∼18.25 MΩ cm) obtained from a Milli-Q synthesis system.Instrumentation
The crystal structures of samples were identified by an X-ray diffraction (XRD) meter (Bruker D8-Advance, Germany). Photocatalytic activity was assessed by the degradation of RhB dye photodegradation under visible light irradiation (18 W λ ≥ 400 nm). UV-visible absorption spectra were obtained through the diffuse reflection method using a spectrometer (HITACHI U-4100, Japan Hitachi). The morphologies of the samples were investigated by scanning electron microscopy (SEM) (QUANTA 250 FEG).Synthesis of samples
g-C3N4 powders were synthesized according to the method in literature.19 Typically, 3 g of melamine was put into a quartz boat and heated in a rate of 5 °C min−1 to 550 °C in a tube furnace and then kept at this temperature for 3 h. All the experiments were performed in air conditions. The resulting yellow product was collected and ground into powder for further use. The typical preparation procedure of Ag2O/g-C3N4 composite photocatalysts was as follows: 0.05 g of g-C3N4 was added into 25 mL of H2O and sonicated with stirring for 2 h. Then, 0.25 g of NaOH was added into the suspension and stirred for 30 min. Further, different amounts of 0.1 M AgNO3 were added drop by drop under stirring, and the mixture was held in the dark for 30 min with continuous stirring. All the experiments were carried out at room temperature. The obtained precipitate was collected by centrifugation and washed with distilled water for four times. Finally, the solid product was dried at 60 °C overnight. A series of Ag2O/g-C3N4 composites with different mass ratios of Ag2O and g-C3N4 were prepared by changing the amounts of g-C3N4 and marked as 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. As a reference, Ag/g-C3N4 composite photocatalyst was prepared using ethanol instead of H2O and kept other conditions same. A series of Ag/g-C3N4 composites with different mass ratios of Ag and g-C3N4 were marked as 1
20. As a reference, Ag/g-C3N4 composite photocatalyst was prepared using ethanol instead of H2O and kept other conditions same. A series of Ag/g-C3N4 composites with different mass ratios of Ag and g-C3N4 were marked as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30.
30.
For preparing BiOBr/g-C3N4 heterojunctions, typically, 0.05 g of g-C3N4 was added into 20 mL of H2O followed by addition of 0.081 g of KBr, and the mixture was ultrasonicated for 1 h to obtain a uniform suspension (suspension A). 0.08 g of Bi(NO3)3·5H2O was dissolved in 10 mL of aqueous solution containing 1 mL of acetic acid (HAc) and vigorously stirred for 30 min (solution B). The solution B was added dropwise into the suspension A and stirred magnetically for 1 h at room temperature. After aging for 6 h, the precipitates were collected by centrifugation, washed thoroughly with distilled water and ethanol, and then dried at 60 °C overnight to get the resulting samples. The different weight ratios of BiOBr/g-C3N4 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were obtained. The pure BiOBr was synthesized using same conditions in the absence of g-C3N4.
4 were obtained. The pure BiOBr was synthesized using same conditions in the absence of g-C3N4.
Photo-catalytic investigation
The photo-catalytic activity of the catalyst was tested by the decomposition of RhB solution. A RhB solution with the concentration of 10 mg L−1 (pH = 5.25) was first prepared. In each test, about 0.01 g of catalyst was added to ∼25 mL of the RhB solution and sonicated for 10 min. Then, the suspension was magnetically stirred for 30 min achieving an adsorption–desorption balance. The degradation process was performed under the UV illumination. At the appropriate reaction time (15 min, 30 min, 1 h, 2 h, 3 h), the 2 mL liquid was taken out. Then, the supernate were isolated from the solution by centrifugation. The concentration of the RhB was determined by monitoring the changes in the maximal absorbance at approximately λ = 554 nm characterized by an UV-vis spectrometer.The active species capture experiments were employed to study the photocatalysis mechanism. At the beginning, 10.0 mM CH3OH (˙OH quencher), TEA (h+ quencher) and PBQ (˙O2− quencher) were added to the RhB aqueous solution.20,21 Then the remaining experimental processes were similar to that of photocatalysis.
Results and discussion
The composition and morphology of as-prepared catalysts were characterized by XRD and SEM analysis. As shown in Fig. 1a, the XRD patterns illustrate the crystalline phase evolution of the as-prepared Ag2O/g-C3N4 composites with different mass ratios. An apparent diffraction peak at 27.4° in pure g-C3N4 were indexed to the (002) planes of hexagonal g-C3N4 (JCPDS 87-1526) arising from the stacking of the conjugated aromatic system corresponding to an interlayer distance of d = 0.33 nm, while the peak at 13.1° represents in-plane structural packing motif with a period of 0.675 nm.22 In the Ag2O/g-C3N4 composite, the diffraction peaks except at 2θ of 27.4° were attributed to the cubic crystal phase (JCPDF 41-1104),5 and a weak diffraction peak of g-C3N4 at 27.4° was found out owing to the relatively low percentage of g-C3N4 (Fig. 1a). With the growth of g-C3N4 content (>50%), the weak peak of Ag2O at 26.7° may be covered by the strong peak of g-C3N4 at 27.4° and cannot be observed in the XRD patterns. The XRD patterns of the as-prepared Ag/g-C3N4 composites with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 are shown in Fig. 1a, the diffraction peaks at 2θ of 38.1°, 44.2°, 64.4° and 77.4° are attributed to the respective (111), (200), (220) and (311) planes of the crystal phase of Ag (JCPDF 04-0783).23 Fig. 1b shows the XRD patterns of the BiOBr and BiOBr/g-C3N4 heterostructured composites. The typical diffraction peaks at 2θ of 10.9°, 21.9°, 25.1°, 31.7°, 32.2°, 39.4°, 46.2° and 57.1° in BiOBr/g-C3N4 sample are ascribed to (001), (002), (101), (102), (110), (112), (200) and (212) facets which can be indexed to the tetragonal phase of BiOBr (JCPDF no. 09-0393) except a typical diffraction peak of g-C3N at 27.4°.
30 are shown in Fig. 1a, the diffraction peaks at 2θ of 38.1°, 44.2°, 64.4° and 77.4° are attributed to the respective (111), (200), (220) and (311) planes of the crystal phase of Ag (JCPDF 04-0783).23 Fig. 1b shows the XRD patterns of the BiOBr and BiOBr/g-C3N4 heterostructured composites. The typical diffraction peaks at 2θ of 10.9°, 21.9°, 25.1°, 31.7°, 32.2°, 39.4°, 46.2° and 57.1° in BiOBr/g-C3N4 sample are ascribed to (001), (002), (101), (102), (110), (112), (200) and (212) facets which can be indexed to the tetragonal phase of BiOBr (JCPDF no. 09-0393) except a typical diffraction peak of g-C3N at 27.4°.
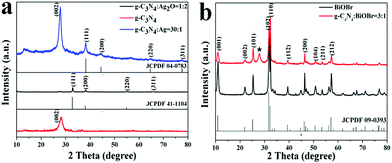 | ||
| Fig. 1 XRD patterns of as-prepared samples: g-C3N4, Ag2O/g-C3N4, Ag/g-C3N4 (a) and BiOBr/g-C3N4. (★: g-C3N4) composites (b). | ||
After coupling with BiOBr, the intensity of dominate (002) peak in g-C3N4 and (001) peak in BiOBr decreased significantly in the composites compared with the separate sample. This suggests that BiOBr has been successfully deposited onto the surface of g-C3N4. The (001) peak of BiOBr in the composite was much wider than that of pure BiOBr, suggesting a stepwise thickness decrease of BiOBr in the heterojunctions, which implies that the crystallite growth of BiOBr along the c-axis would be attenuated after being deposited onto g-C3N4. The results above show that either p–n heterostructure composites or noble metal modified composites are successfully obtained and all exhibit good crystallization.
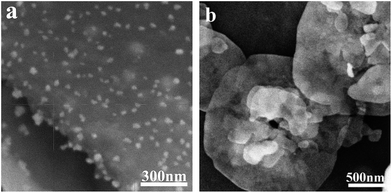
Fig. 2 shows the SEM images of Ag2O/g-C3N4 and the BiOBr/g-C3N4 photocatalysts with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 2a) and 1
2 (Fig. 2a) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 2b). The as-prepared g-C3N4 sample revealed a clearly lamellar structure. After the introduction of Ag2O, many Ag2O particles were evenly dispersed on the surface of g-C3N4. With the mass ratio of g-C3N4 and Ag2O varying from 1
3 (Fig. 2b). The as-prepared g-C3N4 sample revealed a clearly lamellar structure. After the introduction of Ag2O, many Ag2O particles were evenly dispersed on the surface of g-C3N4. With the mass ratio of g-C3N4 and Ag2O varying from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 20
2 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the average size of Ag2O nanoparticles decreased from about 32 nm to 10 nm. The agglomeration can be emerged in pure Ag2O. The addition of g-C3N4 can greatly improve the dispersibility of Ag2O particles and efficiently decrease the particle size of Ag2O. It may be attributed that g-C3N4 with a layer structure offered numerous nucleation sites to the growth of Ag2O particles, leading to the homogeneous dispersion of Ag2O particles on the surface of g-C3N4 with smaller size. All the Ag2O particles are attached to the surface of g-C3N4 so that strong interface interaction could be formed between Ag2O particles and g-C3N4. As reported in literature, the interplanar spacing of the Ag2O/g-C3N4 composites prepared by a simple liquid phase reaction was 0.27 nm corresponded to the (111) plane of Ag2O.20 Fig. 2b clearly shows that there exist two kinds of nanosheets. The flat nanosheets can be assigned to BiOBr and the other small-size ones can be indexed to g-C3N4. On the basis of the crystal structure of BiOBr revealed by XRD patterns, the (BiO)+ layers were interlaid by double slabs of Br−. Such internal structure would control the growth of BiOBr at a certain axis direction to form 2D nanosheet morphology. The BiOBr nanosheets were deposited on the surface of g-C3N4 by situ self-assembled process, resulting in formation of strong interaction between BiOBr and g-C3N4. The large and closely contacted interface in Ag2O/g-C3N4 and the BiOBr/g-C3N4 composites is favorable for charge transfer between two components.
1, the average size of Ag2O nanoparticles decreased from about 32 nm to 10 nm. The agglomeration can be emerged in pure Ag2O. The addition of g-C3N4 can greatly improve the dispersibility of Ag2O particles and efficiently decrease the particle size of Ag2O. It may be attributed that g-C3N4 with a layer structure offered numerous nucleation sites to the growth of Ag2O particles, leading to the homogeneous dispersion of Ag2O particles on the surface of g-C3N4 with smaller size. All the Ag2O particles are attached to the surface of g-C3N4 so that strong interface interaction could be formed between Ag2O particles and g-C3N4. As reported in literature, the interplanar spacing of the Ag2O/g-C3N4 composites prepared by a simple liquid phase reaction was 0.27 nm corresponded to the (111) plane of Ag2O.20 Fig. 2b clearly shows that there exist two kinds of nanosheets. The flat nanosheets can be assigned to BiOBr and the other small-size ones can be indexed to g-C3N4. On the basis of the crystal structure of BiOBr revealed by XRD patterns, the (BiO)+ layers were interlaid by double slabs of Br−. Such internal structure would control the growth of BiOBr at a certain axis direction to form 2D nanosheet morphology. The BiOBr nanosheets were deposited on the surface of g-C3N4 by situ self-assembled process, resulting in formation of strong interaction between BiOBr and g-C3N4. The large and closely contacted interface in Ag2O/g-C3N4 and the BiOBr/g-C3N4 composites is favorable for charge transfer between two components.
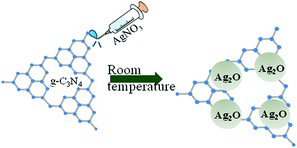
Scheme 1 shows the formation process of Ag2O/g-C3N4. Bulk graphitic carbon nitride was synthesized from melamine via the process of high temperature polymerization. After adding NaOH solution, OH− ions can be attached to the surface of g-C3N4. Ag+ ions with positive charges react with hydroxyl ions to form Ag(OH) precipitates in an aqueous solvent. Ag(OH) precipitates are unstable prone to decomposition to generate Ag2O which deposited on the surface of g-C3N4. However, Ag nanoparticles were dispersed on the surface of g-C3N4 when ethanol was used as solvent instead of aqueous solvent. Silver ions react with hydroxyl ions and ethanol to produce elemental silver under alkaline conditions. The reason for two different composites is that hydroxyl groups of ethanol exhibit weak reduction properties which could reduce silver ions into elemental silver.
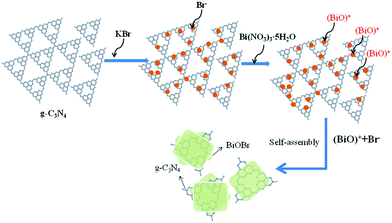
The schematic illustration of the formation process for 2D BiOBr/g-C3N4 heterostructure nanocomposites is presented in Scheme 2. In g-C3N4 aqueous solution, a large number of bromine ions are adsorbed on the surface of g-C3N4 nanosheets due to the electrostatic interactions. After adding bismuth source solution, (BiO)+ ion ionized by the Bi(NO3)3 solution rapidly combines with Bi− to form BiOBr nanosheets and the nanosheets grow along the surface of g-C3N4 nanosheets, which results in the formation of BiOBr/g-C3N4 2D nanojunctions. On account of lamellar structure of g-C3N4, Br− is adsorbed on the (002) crystal plane.24 By means of in situ growth process, BiOBr/g-C3N4 heterostructure composites have stacked layers of laminated structure through self-assembly. Basing on the above mechanisms, there is strong interface interaction existed between two components in heterostructure photocatalysts, which brings more effective interface transfer of photogenerated carriers.
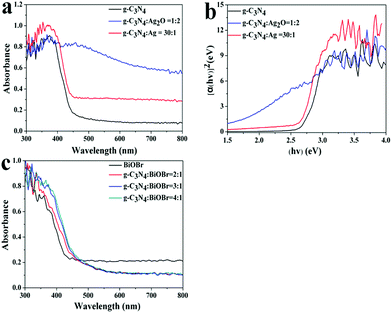
The optical properties of the as-prepared g-C3N4, Ag2O/g-C3N4, Ag/g-C3N4 and the BiOBr/g-C3N4 composites were measured via the UV-vis diffuse reflection technique as shown in Fig. 3. Fig. 3a shows the absorption edge of the pure g-C3N4 is at about 460 nm, which originates from its band gap of 2.7 eV and is consistent with the reported results.4 After the introduction of Ag2O or Ag nanoparticles on the surface of g-C3N4, the optical absorption of the composites in the visible region increases. With the increasing mass ratios of Ag2O and g-C3N4, the absorption intensity of these composites is strengthened. We could calculate the band edge according to the equation: ahν = A(αhν − Eg)n/2.25 Among all the samples of Ag2O/g-C3N4, the band gap of the composite with the mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is the narrowest with about 2.2 eV. The band gap of Ag/g-C3N4 composites with the increasing mass ratio of 1
1 is the narrowest with about 2.2 eV. The band gap of Ag/g-C3N4 composites with the increasing mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 is about 2.6 eV. As expected, the BiOBr sample shows its fundamental absorption edge rising at 430 nm, consistent with the reported results. Fig. 3c shows that the BiOBr/g-C3N4 exhibits a gradual red shift of band edge as the amount of g-C3N4 increased in comparison with BiOBr, which favors the absorption of visible light. The results from UV-vis absorption spectra suggest that the fabrication of the heterostructured composites can greatly improve the optical absorption property and increase the utilized efficiency of solar light, which are favorable for the enhancement of the photocatalytic activity.
30 is about 2.6 eV. As expected, the BiOBr sample shows its fundamental absorption edge rising at 430 nm, consistent with the reported results. Fig. 3c shows that the BiOBr/g-C3N4 exhibits a gradual red shift of band edge as the amount of g-C3N4 increased in comparison with BiOBr, which favors the absorption of visible light. The results from UV-vis absorption spectra suggest that the fabrication of the heterostructured composites can greatly improve the optical absorption property and increase the utilized efficiency of solar light, which are favorable for the enhancement of the photocatalytic activity.
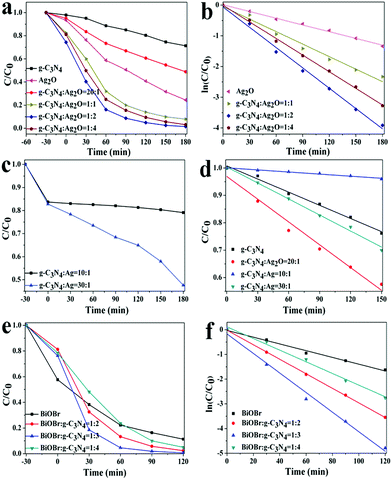
The photocatalytic activities of the as-prepared Ag2O/g-C3N4, Ag/g-C3N4 and BiOBr/g-C3N4 composites with various mass ratios were evaluated by the photodegradation of RhB dye under visible light irradiation shown in Fig. 4. For comparison, the photocatalytic activities of pure g-C3N4, Ag2O and BiOBr were also obtained under the same conditions. The removal percentage of RhB dye was nearly 29% by pure g-C3N4. According to the tendency of degradation, to quantitatively determine the reaction kinetics of RhB dye photodegradation by the as-prepared samples, the experimental data of Ag2O/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), pure BiOBr and BiOBr/g-C3N4 composites were fitted with the pseudo-first-order model, ln(C0/C) = kt, where k was the apparent first-order rate constant, C0 is the initial concentration of RhB solution, C is the concentration of RhB solution at time t, and k is kinetic constant.25 The corresponding kinetic constants (k) were calculated and given in Fig. 4b, d and f. The experimental data of Ag/g-C3N4, Ag2O/g-C3N4 (1
1), pure BiOBr and BiOBr/g-C3N4 composites were fitted with the pseudo-first-order model, ln(C0/C) = kt, where k was the apparent first-order rate constant, C0 is the initial concentration of RhB solution, C is the concentration of RhB solution at time t, and k is kinetic constant.25 The corresponding kinetic constants (k) were calculated and given in Fig. 4b, d and f. The experimental data of Ag/g-C3N4, Ag2O/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and pure g-C3N4 were fitted with the zero-order kinetics model, which may attributed to the extremely high content of g-C3N4 in composite systems. The kinetic model for degradation of composites tend to be fitted with first-order model as the less content of g-C3N4, which is close to the reaction kinetics rules of photodegradating RhB by pure Ag2O or BiOBr photocatalyst. In zero-order kinetics reaction, the degradation rates have nothing to do with concentration of g-C3N4 and Ag2O/g-C3N4 (1
20) and pure g-C3N4 were fitted with the zero-order kinetics model, which may attributed to the extremely high content of g-C3N4 in composite systems. The kinetic model for degradation of composites tend to be fitted with first-order model as the less content of g-C3N4, which is close to the reaction kinetics rules of photodegradating RhB by pure Ag2O or BiOBr photocatalyst. In zero-order kinetics reaction, the degradation rates have nothing to do with concentration of g-C3N4 and Ag2O/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) in RhB solution. When the ratio of Ag2O/g-C3N4 (0.02204 min−1) was 2
20) in RhB solution. When the ratio of Ag2O/g-C3N4 (0.02204 min−1) was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the rate constant of the as-prepared composites was approximately 12 times higher than that of g-C3N4 (0.00183 min−1) and 3 times higher than that of pure Ag2O (0.00732 min−1) under the same conditions. However, among all as-prepared heterostructure composites, BiOBr/g-C3N4 (0.04 min−1) with the mass ratio of 1
1, the rate constant of the as-prepared composites was approximately 12 times higher than that of g-C3N4 (0.00183 min−1) and 3 times higher than that of pure Ag2O (0.00732 min−1) under the same conditions. However, among all as-prepared heterostructure composites, BiOBr/g-C3N4 (0.04 min−1) with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 exhibits the highest photocatalytic activities and RhB solution could be completely degraded within 2 h. We could conclude that the as-prepared composites which meet the pseudo-first-order model are more ideal photocatalysts.
3 exhibits the highest photocatalytic activities and RhB solution could be completely degraded within 2 h. We could conclude that the as-prepared composites which meet the pseudo-first-order model are more ideal photocatalysts.
The enhanced photocatalytic activities of the as-prepared Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and BiOBr/g-C3N4 (1
1) and BiOBr/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) composites which may attribute to the formation of heterostructure between two components which enhance the separation and transfer of charge carriers at the interface. Owing to the formation of type II heterojunction, BiOBr/g-C3N4 (1
3) composites which may attribute to the formation of heterostructure between two components which enhance the separation and transfer of charge carriers at the interface. Owing to the formation of type II heterojunction, BiOBr/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) composites exhibit the best photocatalytic activity, which promotes more effective separation and transfer of photogenerated carriers. Further adjusting the ratio of g-C3N4 and Ag2O to 1
3) composites exhibit the best photocatalytic activity, which promotes more effective separation and transfer of photogenerated carriers. Further adjusting the ratio of g-C3N4 and Ag2O to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, the slightly decreased photocatalytic activity may be attributed to the fact that the higher content of Ag2O may easily result in the agglomerate of Ag2O particles causing a low dispersibility on the surface of g-C3N4. In Ag2O/g-C3N4 (2
4, the slightly decreased photocatalytic activity may be attributed to the fact that the higher content of Ag2O may easily result in the agglomerate of Ag2O particles causing a low dispersibility on the surface of g-C3N4. In Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites, the intimate junctions between g-C3N4 and Ag2O bring some synergistic reaction to enhance the photocatalytic activity. With respect to the Ag/g-C3N4 composites, the improved dispersibility and the decreased particle size of Ag nanoparticles of the as-prepared composites also play important roles in enhancing photocatalytic activity. With the mass ratio of g-C3N4 and Ag increasing from 30
1) composites, the intimate junctions between g-C3N4 and Ag2O bring some synergistic reaction to enhance the photocatalytic activity. With respect to the Ag/g-C3N4 composites, the improved dispersibility and the decreased particle size of Ag nanoparticles of the as-prepared composites also play important roles in enhancing photocatalytic activity. With the mass ratio of g-C3N4 and Ag increasing from 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 10
1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the worse photocatalytic activity can be ascribed to the fact that the higher content of Ag may result in self-nucleation growth of Ag particles hindering rapid separation of photogenerated charge carriers. This may influence the transfer of photogenerated charge carriers as well as the separation efficiency of the photoinduced electron–hole pairs. When the ratio of BiOBr/g-C3N4 and Ag/g-C3N4 composites is 1
1, the worse photocatalytic activity can be ascribed to the fact that the higher content of Ag may result in self-nucleation growth of Ag particles hindering rapid separation of photogenerated charge carriers. This may influence the transfer of photogenerated charge carriers as well as the separation efficiency of the photoinduced electron–hole pairs. When the ratio of BiOBr/g-C3N4 and Ag/g-C3N4 composites is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, samples exhibited poor photocatalytic activities. High content of Ag may impede the transfer of photogenerated from g-C3N4 to Ag. When the content of BiOBr is more than twice as much as that of g-C3N4, BiOBr could be inspired to produce photogenerated electrons and holes, which limits the transfer of photogenerated electrons from g-C3N4 to BiOBr. Although BiOBr is favorable for separation of photogenerated hole–electron pairs, more BiOBr in the composites might reduce the photoabsorption of visible light. Therefore, in heterostructure photocatalysts, a suitable ratio between two components is significant for effectively enhancing the photocatalytic activity. Moreover, it is clearly that type II heterostructure semiconductor nanocomposites show better interface interaction.
4, samples exhibited poor photocatalytic activities. High content of Ag may impede the transfer of photogenerated from g-C3N4 to Ag. When the content of BiOBr is more than twice as much as that of g-C3N4, BiOBr could be inspired to produce photogenerated electrons and holes, which limits the transfer of photogenerated electrons from g-C3N4 to BiOBr. Although BiOBr is favorable for separation of photogenerated hole–electron pairs, more BiOBr in the composites might reduce the photoabsorption of visible light. Therefore, in heterostructure photocatalysts, a suitable ratio between two components is significant for effectively enhancing the photocatalytic activity. Moreover, it is clearly that type II heterostructure semiconductor nanocomposites show better interface interaction.
From the viewpoint of practical applications, the stabilities of the Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composites and BiOBr/g-C3N4 (1
1) composites and BiOBr/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) heterojunctions were further investigated by recycling the photocatalyst for degrading RhB under visible light irradiation. As shown in Fig. 5, the as-prepared two kinds of heterostructure composites show good catalytic stability, maintaining a similar level of reactivity after four cycles. The slight decrease should derive from the inevitable loss of catalyst during the recycling process. In fact, in each cycle of tests, the RhB solution can be almost completely decolored after irradiation for 180 min. In addition, the present photocatalyst can be easily separated from the aqueous system by a simple sedimentation, due to its large size. Thus, the Ag2O/g-C3N4 (2
3) heterojunctions were further investigated by recycling the photocatalyst for degrading RhB under visible light irradiation. As shown in Fig. 5, the as-prepared two kinds of heterostructure composites show good catalytic stability, maintaining a similar level of reactivity after four cycles. The slight decrease should derive from the inevitable loss of catalyst during the recycling process. In fact, in each cycle of tests, the RhB solution can be almost completely decolored after irradiation for 180 min. In addition, the present photocatalyst can be easily separated from the aqueous system by a simple sedimentation, due to its large size. Thus, the Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and BiOBr/g-C3N4 (1
1) and BiOBr/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) heterojunctions described in this paper hold extraordinarily high stability and recyclability for photocatalytic applications.
3) heterojunctions described in this paper hold extraordinarily high stability and recyclability for photocatalytic applications.
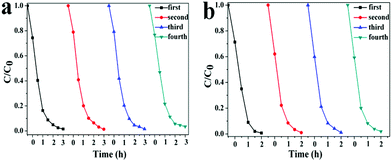 | ||
Fig. 5 Circling runs in the presence of the Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite (a) and BiOBr/g-C3N4 (1 1) composite (a) and BiOBr/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (b) for photodegradation of RhB dye under visible light irradiation. 3) (b) for photodegradation of RhB dye under visible light irradiation. | ||
On the basic of the results above, possible photocatalytic mechanisms of the as-prepared Ag2O/g-C3N4 and BiOBr/g-C3N4 composites under visible light irradiation were illustrated in Scheme 3. As a n-type semiconductor material, g-C3N4 can construct p–n heterostructure with Ag2O (Scheme 3a). Both Ag2O and g-C3N4 can be excited to generate electrons (e−) and holes (h+) under visible light irradiation. In the common p–n type II junctions, owning to the inner electric field if the photogenerated electrons tended to transfer from g-C3N4 to Ag2O, the photogenerated holes had an opposite transfer.26 However, the conduction band (CB) bottom of Ag2O is more positive than that of g-C3N4, while the valence band (VB) top of Ag2O is more negative.27 Because of the inner electric field, the transfer of photogenerated electrons from Ag2O to g-C3N4 is partly limited and the transfer of h+ could be accelerated which causes highly efficient separation of photogenerated charge carriers under visible light irradiation. The photogenerated electrons on the CB of g-C3N4 could be captured by oxygen to generate active species (e− + O2 + H+ = HO2 (aq)). In the Ag2O/g-C3N4 composites, ˙OH, H2O2 and O2− exhibit more positive potentials than the VB of Ag2O.28 The holes on the VB of Ag2O could directly decompose the absorbed RhB dyes on the surface of the photocatalysts, which is helpful to limit the recombination of photogenerated electrons and holes.28 As a result, the photogenerated electron–hole pairs can be separated efficiently, which leads to a highly enhanced photocatalytic activity than the pure g-C3N4 and Ag2O. Therefore, we conclude that the enhanced photocatalytic activity of the as-prepared Ag2O/g-C3N4 can be attributed not only to the improved dispersity and the appropriate mass ratio of g-C3N4 and Ag2O as well as the improved optical absorption property arising from the heterostructure, but also to their synergetic effects of matched energy band structure and the inner electric field. All the reasons above can bring the rapid efficient separation of the photogenerated electron–hole pairs.
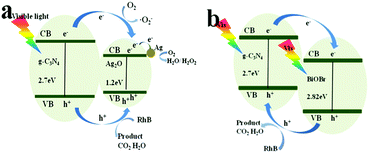 | ||
| Scheme 3 Proposed photocatalytic mechanisms over the Ag2O/g-C3N4 composites (a) and BiOBr/g-C3N4 composites (b). | ||
In the Ag/g-C3N4 composites, on the one hand, the fast generation, rapid separation and transportation of these photogenerated carriers at the interface of g-C3N4 and Ag are the main reason for the enhanced visible-light photocatalytic activity of Ag/g-C3N4. As is reported, the theoretical calculated CB and VB potential of g-C3N4 are about −1.3 and 1.4 V (vs. NHE),29 respectively, while the Fermi level of Ag is 0.4 V (vs. NHE).30 After visible-light irradiating, the generated electrons on g-C3N4 can transfer rapidly to Ag nanoparticles, creating a Schottky barrier that narrows the band gap of g-C3N4. On the other hand, the visible-light harvesting ability of Ag/g-C3N4 is enhanced due to surface plasmon resonance (SPR) absorption of silver nanoparticles. The Ag/g-C3N4 with higher loading of Ag nanoparticles could exhibit stronger SPR effect, but Ag/g-C3N4 with excess Ag nanoparticles show worse photocatalytic activity which may attribute to the light opacity increasing, preventing g-C3N4 from the effective light absorption.
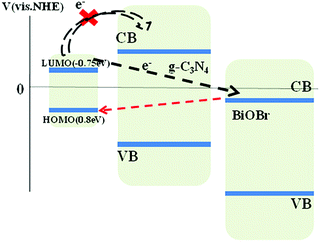
As is shown in Fig. 3b, the band structures of g-C3N4 and BiOBr are well-matched upon their intimately contacted interface and aligned with each other. The relative CB and VB edge positions of g-C3N4 nanosheets and BiOBr nanosheets imply that both the well-matched band energies and crystal planes can form type II heterojunctions at nanoscale level. Both g-C3N4 and BiOBr can be excited by visible light and the exited electrons produced by g-C3N4 were injected into the CB of BiOBr. The exited holes produced by BiOBr were injected into the VB of g-C3N4 which results in efficient separation and transport of photo-induced electrons and holes. Both electrons and holes all show extreme activity, which can decompose RhB directly. However, there are some arguments about the effect of photogenerated electrons. On the one hand, owing to RhB is active with light, the dye-sensitised photocatalysis would also exist in this system. In fact, as shown in Scheme 4, dye sensitization process cannot occur because the lowest unoccupied molecular orbital (LUMO) potential of RhB (−0.75 eV) is more positive than the CBM of g-C3N4.31 The excited electrons of RhB cannot transfer to the CB of g-C3N4. On the other hand, as is reported, the conduction band and the valence band of BiOBr are both more positive than the standard redox potentials of O2/O2− and ˙OH/OH−. Even though a successful transfer of electrons from the LUMO of RhB to the CB of BiOBr would take place, these electrons cannot further reduce O2 to form O2− species which would return to the HOMO of RhB, as marked by the dotted line (blue, Scheme 4). We believe that the dye-sensation which would be helpful to degradate RhB in the BiOBr/g-C3N4 system is impossible. Thus, it is expected that the enhanced photocatalytic activity of the BiOBr/g-C3N4 2D nanojunctions can be attributed to the well-matched band structure of type II heterojunctions and the appropriate mass ratio of g-C3N4 and BiOBr as well as the improved optical absorption property and the special morphology of layer-by-layer self-assembly.
On the basis of the above discussion, compared with pure g-C3N4, the enhanced photocatalytic activity of Ag2O/g-C3N4 and BiOBr/g-C3N4 toward the degradation of RhB is explained by the enhanced visible-light utilization efficiency due to the p–n heterostructures between two components. Due to superior type II heterojunction, the photocatalytic activity of BiOBr/g-C3N4 enhanced much more than that of Ag2O/g-C3N4. One of the main reasons is that, under visible light irradiation, type II heterojunction photocatalysts can achieve more rapidly generate, separate of photogenerated charge carriers and participate in photocatalytic degradation of RhB, where electrons and holes play significant roles to degrade organic dyes. However, as a type I heterostructure, photogenerated electrons and holes of Ag2O/g-C3N4 are inevitably likely to recombine on the surface of Ag2O which affect to photocatalytic activity. Although the surface modification of noble metals and surface plasmon resonance effect can contribute to higher the photocatalytic activity in Ag/g-C3N4 composites, structure defects still remain in bulk g-C3N4 which limits the efficient separation of photogenerated charge carriers. There is no doubt that semiconductor composites photocatalysts with p–n heterostructure can be more able to improve the photocatalytic activity than noble metals modified composites.
Generally, photoinduced reactive species including trapped holes, ˙OH radicals, and O2˙− are expected to be involved in the photocatalytic process. To prove the photocatalytic mechanism, the main reactive species in the photocatalytic process were detected through the trapping experiments of radicals using CH3OH as the hydroxyl radical scavenger, p-benzoquinone (PBQ) as the superoxide radical scavenger, and triethanolamine (TEA) as the hole radical scavenger.
Fig. 6a shows that the degradation efficiency of RhB in the Ag2O/g-C3N4 composites (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) decreased obviously with the addition of a hydroxyl radical scavenger (CH3OH; the conversion of RhB was 98.6%), which reduced slightly with the addition of a superoxide radical scavenger (PBQ; the conversion of RhB was 24%) and reduced mostly with the addition of a hole radical scavenger (TEA; the conversion of RhB was 54.3%). This results indicate that superoxide radicals were the main reactive active species and the hole radicals were secondary reactive active species. As is shown in Fig. 6b, under visible light irradiation photocatalytic activities of BiOBr/g-C3N4 composites (1
1) decreased obviously with the addition of a hydroxyl radical scavenger (CH3OH; the conversion of RhB was 98.6%), which reduced slightly with the addition of a superoxide radical scavenger (PBQ; the conversion of RhB was 24%) and reduced mostly with the addition of a hole radical scavenger (TEA; the conversion of RhB was 54.3%). This results indicate that superoxide radicals were the main reactive active species and the hole radicals were secondary reactive active species. As is shown in Fig. 6b, under visible light irradiation photocatalytic activities of BiOBr/g-C3N4 composites (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) decreased slightly after addition of TEA (the conversion was 19.2%), which means holes radicals were the main active species.
3) decreased slightly after addition of TEA (the conversion was 19.2%), which means holes radicals were the main active species.
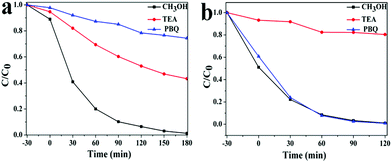 | ||
Fig. 6 Plots of photogenerated carrier trapping during RhB degradation over the Ag2O/g-C3N4 composite (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (a) and BiOBr/g-C3N4 composites (1 1) (a) and BiOBr/g-C3N4 composites (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (b) under visible light irradiation. 3) (b) under visible light irradiation. | ||
Conclusions
In this paper, a series of Ag2O/g-C3N4, Ag/g-C3N4, and BiOBr/g-C3N4 heterostructured photocatalysts with different mass ratios of g-C3N4 and Ag2O were prepared by a simple liquid phase reaction process at room temperature. The photodegradation of RhB experiments revealed that the BiOBr/g-C3N4 heterostructured composite with the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 degraded nearly 100% of RhB within 2 h, while Ag2O/g-C3N4 (2
2 degraded nearly 100% of RhB within 2 h, while Ag2O/g-C3N4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) degraded thoroughly within 3 h. The degradation rate constant of BiOBr/g-C3N4 (1
1) degraded thoroughly within 3 h. The degradation rate constant of BiOBr/g-C3N4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was 0.04 min−1 under visible light, which was almost 2 and 14 times more than that of Ag2O/g-C3N4 and Ag/g-C3N4, respectively. In composite systems, the reaction kinetics of RhB dye photo-degradation of Ag2O/g-C3N4 and BiOBr/g-C3N4 composites were fitted with the pseudo-first-order model, ln(C0/C) = kt, while the experimental data of Ag/g-C3N4 and pure g-C3N4 were fitted with the zero-order kinetics model. Then, we conclude that the as-prepared samples which meet the pseudo-first-order model are optimal photocatalysts. The results provided here not only offer a highly efficient and stable photocatalytic material for environmental remediation, but also showed a new insight for constructing efficient heterostructured photocatalysts.
3) was 0.04 min−1 under visible light, which was almost 2 and 14 times more than that of Ag2O/g-C3N4 and Ag/g-C3N4, respectively. In composite systems, the reaction kinetics of RhB dye photo-degradation of Ag2O/g-C3N4 and BiOBr/g-C3N4 composites were fitted with the pseudo-first-order model, ln(C0/C) = kt, while the experimental data of Ag/g-C3N4 and pure g-C3N4 were fitted with the zero-order kinetics model. Then, we conclude that the as-prepared samples which meet the pseudo-first-order model are optimal photocatalysts. The results provided here not only offer a highly efficient and stable photocatalytic material for environmental remediation, but also showed a new insight for constructing efficient heterostructured photocatalysts.
Acknowledgements
This work was supported in part by the program for Taishan Scholars, the projects from National Natural Science Foundation of China (Grant no. 51572109, 51501071, 51302106, 51402123, and 51402124).References
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206 RSC
.
- S. Liu, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914 CrossRef CAS PubMed
.
- S. T. Kochuveedu, D. P. Kim and D. H. Kim, J. Phys. Chem. C, 2012, 116, 2500 CAS
.
- J. Zhang, X. Chen, K. Takanabe, K. Maeda, K. Domen, J. D. Epping, X. Fu, M. Aatonietti and X. Wang, Angew. Chem., Int. Ed., 2010, 49, 441 CrossRef CAS PubMed
.
- M. Xu, L. Han and S. Dong, ACS Appl. Mater. Interfaces, 2013, 5, 12533 CAS
.
- Y. Tian, B. Chang, J. Lu, J. Fu, F. Xi and X. Dong, ACS Appl. Mater. Interfaces, 2013, 5, 7079 CAS
.
- C. Han, L. Ge, C. Chen, Y. Li, X. Xiao, Y. Zhang and L. Guo, Appl. Catal., B, 2014, 147, 546 CrossRef CAS
.
- L. Xu, J. Xia and H. Xu, J. Power Sources, 2014, 245, 866 CrossRef CAS
.
- Z. Chen, P. Sun, B. Fan, Z. Zhang and X. Fang, J. Phys. Chem. C, 2014, 118, 7801 CAS
.
- T. Zhu, Y. Song, H. Ji, Y. Xu, Y. Song, J. Xia, S. Yin, Y. Li, H. Xu, Q. Zhang and H. Li, Chem. Eng. J., 2015, 271, 96 CrossRef CAS
.
- K. Shen, M. A. Gondal, R. G. Siddique, S. Shi, S. Wang, J. Sun and Q. Xu, Chin. J. Catal., 2014, 35, 78 CrossRef CAS
.
- L. M. Lyu and M. H. Huang, J. Mater. Sci., 2014, 49, 5309 CrossRef
.
- D. Sarkar, C. K. Ghosh, S. Mukherjee and K. K. Chattopadhyay, ACS Appl. Mater. Interfaces, 2012, 5, 331 Search PubMed
.
- Y. Yang, Y. Guo, F. Liu, X. Yuan, Y. Guo, S. Zhang, W. Guo and M. Huo, Appl. Catal., B, 2013, 142, 828 CrossRef
.
- J. M. Song, C. J. Mao, H. L. Niu, Y. H. Shen and S. Y. Zhang, CrystEngComm, 2010, 12, 3875 RSC
.
- L. Kong, Z. Jiang, T. Xiao, L. Lu, M. O. Jones and P. P. Edwards, Chem. Commun., 2011, 47, 5512 RSC
.
- Y. Li, Y. Liu, J. Wang, E. Uchaker, Q. Zhang, S. Sun, Y. Huang, J. Li and G. Cao, J. Mater. Chem. A, 2013, 1, 7949 CAS
.
- S. Shenawi-Khalil, V. Uvarov, S. Fronton, I. Popov and Y. Sasson, J. Phys. Chem. C, 2012, 116, 11004 CAS
.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397 CrossRef CAS PubMed
.
- H. T. Ren, S. Y. Jia, Y. Wu, S. H. Wu, T. H. Zhang and X. Han, Ind. Eng. Chem. Res., 2014, 53, 17645 CrossRef CAS
.
- K. Li, S. Guo, Q. Wang, H. Xu, Z. Wang, B. Huang, Y. Dai and J. Lu, ACS Appl. Mater. Interfaces, 2015, 7, 9023–9030 CAS
.
- P. Niu, L. Zhang, G. Liu and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS
.
- C. Su, L. Liu, M. Zhang, Y. Zhang and C. Shao, CrystEngComm, 2012, 14, 3989 RSC
.
- Y. Sun, W. Zhang, T. Xiong, Z. Zhao, F. Dong and R. Wang, J. Colloid Interface Sci., 2014, 418, 317 CrossRef CAS PubMed
.
- Y. Liu, J. Wang, P. Yang and K. Matras-Postolek, RSC Adv., 2015, 5, 61657 RSC
.
- L. Li, L. Xu, W. Shi and J. Guo, Int. J. Hydrogen Energy, 2013, 38, 816 CrossRef CAS
.
- S. C. Yan, S. B. Lv, Z. S. Li and Z. G. Zou, Dalton Trans., 2010, 39, 1488 RSC
.
- X. Wang, S. Li, H. Yu, J. Yu and S. Liu, Chem.–Eur. J., 2011, 17, 7777 CrossRef CAS PubMed
.
- X. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596 CrossRef CAS
.
- J. Li, S. K. Cushing, J. Bright, F. Meng, T. R. Senty, P. Zheng, A. D. Bristow and N. Wu, ACS Catal., 2012, 3, 47 CrossRef
.
- H. Gerischer and F. Willig, Physical and Chemical Applications of Dyestuffs, Springer, Berlin Heidelberg, 1976, vol. 31 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2016 |