Synthesis, structure and photophysical properties of near-infrared 3,5-diarylbenzoBODIPY fluorophores†
Abstract
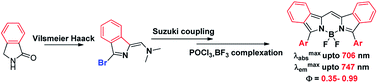
A set of 3,5-diarylated benzoBODIPYs were prepared within three steps from commercial isoindolin-1-one via Vilsmeier Haack, Suzuki coupling and a one-pot POCl3-promoted self-condensation in concert with a BF3 complexation. These benzoBODIPYs showed strong absorption and intense fluorescence emission in far-red to NIR region (λmaxabs = 634–706 nm, λmaxem = 663–747 nm, Φ = 0.35–0.99) tunable via the variation of the 3,5-aryl substituents.

 Please wait while we load your content...
Please wait while we load your content...