A simple and effective way to fabricate mechanical robust superhydrophobic surfaces†
Hao Tiana,
Fajun Wang*a,
Sijie Gea,
Junfei Oua,
Wen Lia and
Shijin Yub
aSchool of Materials Science and Engineering, Nanchang Hangkong University, Nanchang 330063, P. R. China. E-mail: jjbxsjz@foxmail.com; Fax: +86-791-86453210; Tel: +86-791-86453210
bSchool of Mechanical and Electronic Engineering, Jingdezhen Ceramic Institute, Jingdezhen 333403, P. R. China
First published on 15th March 2016
Abstract
A simple, inexpensive and effective method is developed to fabricate mechanical robust superhydrophobic (SH) surfaces based on particle-filled silicone rubber (SR) composites. A large variety of particles with different features, such as copper, SiO2, BaTiO3 (BT), carbon black (CB), and polyvinylidene fluoride (PVDF) can be used to prepare the SH surfaces based on SR composites. The particles were spread on the un-cured SR solution (in n-hexane) surface using a sieve. The excessive particles were removed using ultrasonic washing after the SR matrix was completely cured. Surface roughness is formed due to the accumulation of particles, and the SR matrix provides the required low surface energy. Various SH surfaces based on particle-filled SR composites are obtained with high water contact angle (CA, >160°) and low water sliding angle (SA, <10°). In addition, besides the superhydrophobicity, the surface color, transparence, electric conductivity and other properties can also be achieved by carefully selecting appropriate particles. All of the particles are commercial and used as received without any modification. Furthermore, the mechanical stabilities of the SH surfaces are systematically studied using various possible mechanical actions. The results indicate that the SH surface is mechanically robust against sandpaper abrasion (32.5 kPa, 50 cycles), finger touch, brushing and scratching, as well as high-pressure water impacting (0.12 MPa). The SH surfaces based on particle-filled SR composites can be fabricated at large scale without using any expensive materials and special equipment. Therefore, the SH surface could provide a candidate for practical self-cleaning applications.
Introduction
Surfaces with a water contact angle (CA) larger than 150° and a sliding angle (SA) lower than 10° are known as superhydrophobic (SH) surfaces.1–3 SH surfaces have received extensive interest in both fundamental research and engineering applications.4–8 Various potential applications including anti-corrosion,9 self-cleaning,10 anti-icing,11,12 anti-bacterial and so forth have been reported in recent years.13–15 The artificial SH surfaces have been fabricated via various methods, such as etching,16 spraying,17 dip-coating,18 templating,19 sol–gel techniques,20 nanoimprint lithography,21 electrospinning,22 and so on. However, most of these methods have drawbacks, such as complex routes, high cost, the use of expensive materials and specific equipments. Hence, developing simple, low-cost and effective method to fabricate SH surface is highly desirable for its practical application.Another shortcoming of SH surface is the weak mechanical durability, which hinders the real application of SH surfaces.23–26 The micro- and/or nano-structures of the SH surface is brittle. Normal contacts, such as finger touch, water impacting, abrasion and scratching, can cause damages of the SH surfaces, resulting the degradation of the surface superhydrophobicity.27 In addition, the outmost low-surface-energy material can be peeled off by mechanical contact, causing a decline of the SH property. Therefore, great efforts have been made to resolve this problem. For example, an acrylic polyurethane coating with mechanically robust superhydrophobicity was prepared by Xue et al.26 After 200 cycles of abrasion with sandpaper, the coating surface retains a CA larger than 150°. However, the applied pressure is very low (about 2 kPa). A steel surface with mechanical robust superhydrophobicity was prepared via a chemical etching/fluoridation process.16 The surface did not lose its superhydrophobicity after abraded by sandpaper under a pressure of 16 kPa. However, both the corrosive reagents, such as HCl, HNO3 and H2O2, and expensive fluorinating agent (FAS-17) were used during the fabrication process. Mechanical durable SH surfaces were fabricated by a hot pressing method, followed by the deposition of silver and surface fluorination.27 When a pressure of 10 kPa was applied to the SH surface, the surface can retain its superhydrophobicity after 5 abrasion cycles. In addition, the fabrication method is simple and fast. Once damages on the SH surface occurred, the SH surface can be repaired easily. However, the deposition of silver can only be applied to the surface of copper. Additionally, the expensive fluorinating agent (1H,1H,2H,2H-perfluorodecanethiol) was also used. Thought lots of mechanical stable SH surfaces have been reported, the mechanical stabilities were estimated under relatively weak test conditions (e.g., 2 kPa, 2.9 kPa, 10 kPa and 16 kPa).16,26–28 In addition, other normal mechanical contacts that can not be avoided during the normal uses, such as finger touch, water impacting, brushing and scratching, have not been used to evaluate the mechanical stabilities of the SH surfaces. However, the ideal mechanical robust SH surfaces must remain their superhydrophobicity after subjected to various possible mechanical actions.
Room-temperature vulcanized (RTV) silicone rubber (SR) is a silicone-containing polymer elastomer with low surface energy.19,29–31 The commercialized SR coating is hydrophobic and can be used for insulator coatings for the purpose of reducing containments.19 SH surface based on SR have been fabricated by incorporated the SR matrix with various particles, such as ZnO, Al2O3·3H2O and others.29–31 In addition, the template method and CF4 plasma modification were also reported to fabricate SH surface based on SR. However, the mechanical stabilities of these SR based SH surfaces have not been systematically investigated. In this work, we develop a simple, inexpensive and effective method to fabricate SR based SH surface (see Fig. 1). Various particles, including metal powders, oxides, polymer powders, carbon black powders, etc. can be used to fabricate the SR based SH surfaces by spreading the powders on the un-cured SR solution surface. After the SR completely cured, the particle/SR composite surface was washed under ultrasonication in ethanol to remove the excessive particles. All of the particle/SR composites surfaces exhibit excellent superhydrophobicity, with water CA larger than 160° and water SA lower than 10°. The SH surface based on particle-filled SR composites show robust mechanical stabilities, which are testified by various mechanical damage tests, such as finger toughing, scratching, brushing, water impacting and abrasion.
 | ||
| Fig. 1 Schematic diagram of the method used for preparation of superhydrophobic surfaces based on SR composites. | ||
Experimental section
Materials
Copper powders (Cu) were purchased from Shanghai ChaoWei Nanotechnology Co., Ltd. Polyvinylidene Fluoride (PVDF, FR904) powders were purchased from Shanghai 3F Co., Ltd. Silica microparticles (m-SiO2) were purchased from Nanchang Xinghuo Nanotechnology Co., Ltd. Silica nanoparticles (n-SiO2, M-5) and Carbon Black (CB, VULCAN XC-72) were purchased from Cabot Chemical Co., Ltd. Barium titanate (BT) particles were purchased form Hebei Xiongwei Chemical Co., Ltd. Stainless steel sieve (200#) was purchase from Wuhan Metal Products Co., Ltd. Room temperature vulcanized silicone rubber (SR) were purchased from Shanghai Silicone Mountain Macromolecular Materials Co., Ltd, including α,ω-dihydroxy polydimethylsiloxane (viscosity is about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cP), curing agent (tetraethoxysilane) and catalyst (dibutyltin dilaurate). Hexane was obtained from Shanghai Chemical Reagent Co., Ltd.
000 cP), curing agent (tetraethoxysilane) and catalyst (dibutyltin dilaurate). Hexane was obtained from Shanghai Chemical Reagent Co., Ltd.
Preparation
The SH surfaces based on various SR composites were fabricated via a simple process and systematically illustrated in Fig. 1. Flat glass was used as substrate in this work [Fig. 1(a)]. Other substrates, such as stainless steel plate, copper plate, typing paper, abrasive paper, polyethylene plate and ceramic tile could also be used. In a typical fabricating process, α,ω-dihydroxy polydimethylsiloxane (20.0 g), curing agent (1.0 g), catalyst (0.2 g) and n-hexane (30 mL) were mixed in a beaker and stirred for 10 min at room temperature to form a transparent solution (denoted as SR solution hereafter). After degassed under vacuum for about 10 min, the SR solution was cast onto the glass substrate [Fig. 1(b)]. Copper powders (30 g) were carefully spread on the surface of the solution by using a sieve [Fig. 1(c)] until the solution surface was completely covered by the copper powders. The copper powders sunk slowly from the surface to the bottom in the solution during the curing process [Fig. 1(d) and (e)]. The SR could be peeled off easily from the substrate after fully cured at room temperature (about 25 ± 3°) for 24 h [Fig. 1(f)]. After washed with ethanol repeatedly in an ultrasonic cleaner and then dried, the SH SR surface was obtained (denoted as Cu/SR composite). Various SH surfaces based on SR composites could also be fabricated via the same method when different particle were used. In the present work, a variety of particle fillers, including metal powders (Cu), metal oxide powders (m-SiO2, n-SiO2 and BT), polymer powders (PVDF), carbon black (CB) powders, were selected. The corresponding sample was denoted as PVDF/SR, m-SiO2/SR, n-SiO2/SR, BT/SR and CB/SR composite, respectively.Characteristics
Water contact angles (CAs) and sliding angles (SAs) for various samples were measured using a Krüss DSA 100 apparatus (Germany). The nanostructures of CB and n-SiO2 particles were observed using Transmission Electron Microscopy (TEM, FEI Tecnai G20, America). The microstructures of Cu, PVDF, n-SiO2 and BT particles, were observed using Field Emission Scanning Electron Microscope (FE-SEM, NoVaTM Nano SEM 250, FEI). The surface rough microstructures for various SH samples were also analyzed using FE-SEM. The mechanical stability of the SH samples was measured using sandpaper (400#) as abrasion surface and the method was illustrated in Fig. S1 (see ESI).†Results and discussion
The morphologies and particle sizes of various particles (or powders) were measured using FE-SEM or TEM. Fig. 2(a) shows that the Cu particles are spherical and the diameter is about 1–2 μm. Fig. 2(b) shows that the PVDF particles agglomerate together. The typical size of PVDF aggregation is in the range from about 2 to 8 μm. Fig. 2(c) shows that the m-SiO2 particles are spherical and the diameter is about 1–3 μm. Fig. 2(e) shows that the BT particles exhibit a spherical-like appearance with diameter of about 100 nm. The morphologies and sizes of n-SiO2 and CB powders were observed by TEM. It was observed that both of the n-SiO2 and CB powders were agglomerated together. In addition, the primary granule size of n-SiO2 and CB are about 20 and 50 nm, respectively [see Fig. 2(d) and (f)]. | ||
| Fig. 2 FE-SEM images of various particles: (a) Cu particles; (b) PVDF particles; (c) m-SiO2 particles; (e) BT particles. TEM images: (d) n-SiO2 particles and (f) CB particles. | ||
The wettabilities of different particles were measured by measuring the contact angle (CA) of the particles. Briefly, the different types of particle fillers, i.e., Cu, m-SiO2, PVDF, n-SiO2, CB and BT, were carefully spread on the surface of a glass slide by using double faced adhesive tape [DFAT, see Fig. 3(a)–(f)]. For CA measurement, the particle fillers should cover the surface of DFAT completely to eliminate any of the influence of DFAT. One can see that the CAs for the surfaces of Cu, PVDF and CB particles are all larger than 150° [see Fig. 3(b), (c) and (e)], which demonstrates the hydrophobicity of these particle surfaces. It should be noticed that pure metal surface is hydrophilic. However, metal can absorb airborne hydrocarbons spontaneously on its surface. The hydrocarbons possess low surface free energy. Therefore, the Cu particles exhibit superhydrophobicity.32,33 Whereas the CAs for the surfaces of m-SiO2, n-SiO2 and BT particles are hydrophilic, with all the CAs equal to 0° [see Fig. 3(b), (d) and (f)]. Hence, the particles of the three metal oxides possess hydrophilic features. However, after incorporated with the matrix (i.e., SR), all the obtained particle/SR composites exhibit excellent superhydrophobicity. Water droplets show spherical shape on the surfaces of all the particle/SR composites and roll easily. As depicted in Movie S1 in ESI,† water can not wet the samples' surfaces and run away quickly without any residue. Fig. 4 shows the CA and SA measurements for different SR based composites. One can see that all of the SR based composites surfaces possess high CAs (larger than 160°) and low SAs (lower than 10°). Additionally, the SR based composites exhibit different colors. For example, the Cu/SR composite is purple bronze, the BT/SR composite is white and the CB/SR composite is black [see Fig. 4(a), (e) and (f)]. Therefore, we can fabricate different SR based composites with decorative purposes. Colored SH surfaces have been reported in literatures.34–36 However, the mechanical stabilities of these HS surface have not been investigated. Furthermore, the n-SiO2/SR composite shows semi-transparent appearance, which means the SH composite of n-SiO2/SR could be used for the requirements of the pervious to light, such as windows, etc.37,38 Moreover, the surface of CB/SR composite also shows electrical conductivity due to the contact of a great number of CB particles. The CB particles form conductive path across the surface of CB/SR composite, which endow the electrical conductivity of the composite. Hence, the SH surface of CB/SR composite can be used in the field of electrostatic shielding.39,40 It should be mentioned that the SR based SH surfaces are fabricated via a simple, effective and inexpensive method without using any special equipment and expensive reagent. A large variety of particles, including metal, inorganic oxides and polymer, can be used to prepare large scale SH surfaces based on SR without any modification [see Movie S1, ESI†]. All materials are commercial available and used as received. Neither the hydrophobic particles (such as PVDF, CB) nor the hydrophilic particles (such as SiO2 and BaTiO3) can be used directly to fabricate SH surfaces. Furthermore, besides superhydrophobicity, other properties, such as color, transparency, electrical conductivity, etc. can also be achieved by carefully selecting proper particles. For example, incorporating with Fe3O4 micro and/or nano-particles, a black Fe3O4/SR composite surface exhibiting both superhydrophobicity and magnetism could be expected.41,42
 | ||
| Fig. 3 CA measurement on the surfaces of various particles: (a) Cu particles; (b) m-SiO2 particles; (c) PVDF particles; (d) n-SiO2 particles; (e) CB particles and (f) BT particles. | ||
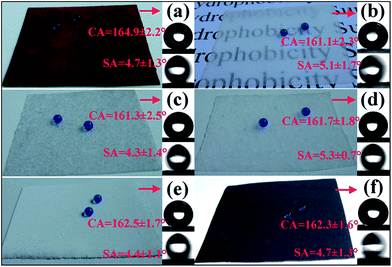 | ||
| Fig. 4 CA measurement for various SR based composites: (a) Cu/SR; (b) n-SiO2/SR; (c) m-SiO2/SR; (d) PVDF/SR; (e) BT/SR and (f) CB/SR. | ||
The surface topographies of different SR based composites were systematically measured using FE-SEM and showed in Fig. 5. A large number of coarse structures characterized by lots of protrusions and pores were formed on the SR surfaces during the curing process. One can see that nearly all of the composites' surfaces are rough because particles and/or particle aggregates covered the SR matrix surfaces completely [see Fig. 5(a), (c), (e), (g), (i) and (k)]. In addition, the SR matrix is a silicone-containing polymer, which possesses low surface energy. A smooth SR surface is hydrophobic with a water contact angle about 95°. Hence, the surface superhydrophobicity of the particles/SR composites could be attributed the combination of the rough structures provided by the particles (and/or particle aggregates) and the low surface energy provided by SR matrix.43,44 The surface particles and/or particle aggregates were anchored firmly by the SR matrix [see Fig. 5(b), (e), (d), (h), (j) and (l)]. The sample was subjected to water impacting under a water pressure of about 0.12 MPa [see Movie S2, ESI†] and then immersed in ethanol under ultrasonic treatment for 30 min. It was observed that neither water impacting nor ultrasonic treatment can peel off the particles from the surface. The surface still remains superhydrophobicity after the above two tests, demonstrating the desired stability of the superhydrophobic surfaces. The water impacting resistance of the SH surfaces have also been investigated in literatures.16,45,46 However, the impacting water pressures applied in their experiments are very low. For example, Yildirim and Wang et al. used water droplets with volume of about 100 μL to impinging the SH surface from 30 cm at a rate of one drop per s.16,46 Seo et al. used a dispenser bottle to jet water onto the SH surface. In our case, a high water pressure (∼0.12 MPa) was applied to impact the SH surface.45 To the best of our knowledge, a SH surface that exhibits mechanical stability against a water impacting pressure as high as 0.12 MPa have not been reported.
Besides water impacting and ultrasonic treatment, the mechanical stabilities of the samples against various normal contacts, such as finger touch, brushing and scratching were also investigated. As showed in Fig. 6 and Movie S3 (see ESI).† The Cu/SR composite surface remains its superhydrophobicity after the above measurement. It is well known that SR is an elastic rubber. The elastic micro-structures on the SR surface can be compressed to avoid destruction by elastic deformation.28 The deformation will recover to its original structures when the external force is withdrawn. Hence, the SR surface possesses excellent mechanical stabilities. As reported in literatures, abrasion test using sandpaper as an abrasive surface was usually applied to evaluate the mechanical stability of a SH surface.16,26–28,43 In our work, the similar abrasion test was also carried out using a home-made method (see ESI, Fig. S1†). As shown in Fig. 7, though small variations of both CAs and SAs are observed, the superhydrophobicity of the samples surface remains unchanged. The CAs are always larger than 155° and the SAs are always lower than 10° even after 50 cycles of abrasion. It should be mentioned that the reported mechanical robust SH surfaces were measured under weak test conditions (e.g., 2 kPa, 2.9 kPa, 10 kPa and 16 kPa).16,26–28 In the present case, a high pressure of ca. 32.5 kPa is applied, which is 2–16 times as high as the reported values. Our SH surface is robust under harsh testing conditions, demonstrating its excellent mechanical stability.
 | ||
| Fig. 7 Mechanical stability measurement for the Cu/SR composite. The abrasion surface is 400# sandpaper. | ||
The influence of abrasion on the surface microstructures of the Cu/SR composite was also measured and showed in Fig. 8. One can see that though the surface microstructures changed after each abrasion cycle, the sample surface is still rough. A great amount of protrusions and pores could be observed on the abraded surface. It has been reported that the SH Teflon surface can be prepared using the abrading method with sandpaper.47 Smooth Teflon is hydrophobic with an average CA of 103°. After roughened by sandpaper, the Teflon surface exhibits superhydrophobic. In the present case, the SR matrix is also hydrophobic, the surface exhibit good abrasion resistance because the fresh exposed surface is also rough with low surface energy. In addition, when 600# sandpaper was used as an abrasion surface under the same pressure of 32.5 kPa, the surface CAs gradually decrease and the SAs gradually increase. After only 20 cycles of abrasion, the sample loses its superhydrophobicity [see ESI, Fig. S3†]. However, after abraded with 400# sandpaper for only 10 cycles, the Cu/SR composite surface exhibits superhydrophobicity again. Carefully investigation indicates that the sandpaper with grade between 280# and 400# can prepare the desired rough structures on the SR composites' surfaces and endow the surfaces with superhydrophobicity.
 | ||
| Fig. 8 FE-SEM microstructures of the Cu/SR composite surface after different abrasion cycles: (a) 0 cycle; (b) 10 cycles; (c) 20 cycles; (d) 50 cycles. The abrasion surface is 400# sandpaper. | ||
Conclusion
SH surfaces were prepared via a simple and inexpensive method. Various particles such as Cu, CB, SiO2, PVDF and BT were used to fabricate SH surface based on SR composite. The particles were spread on the surface of the SR solution. After the curing of SR, the sunken and surface adhered particles were anchored by the SR matrix, while the surface excessive particles were removed by ultrasonic washing. The obtained particle/SR composites' surfaces exhibit excellent superhydrophobicity. The water CAs are larger than 160° and water SAs lower than 10° for all of the particle/SR surfaces. All of the materials are commercial available and used as received. Any expensive reagent and special equipment were not used. Using 12 nm SiO2 particles as filler, the obtained SiO2/SR composite exhibits both surface superhydrophobicity and transparency. Using CB as filler, the CB/SR composite surface is black, superhydrophobic and electrical conductive. Multifunction surfaces based on particle/SR composites can be designed by carefully selecting particle fillers. The particle/SR composites exhibit desired robust mechanical stabilities. The surface retains its superhydrophobicity after various mechanical actions, such as finger touch, scratching, brushing, and high water pressure impacting. An abrasion test indicates that the SH surface can subjected to 50 cycles of abrasion without losing its superhydrophobicity when a high pressure of 32.5 kPa was applied on 400# SiC sandpaper surface. Compared with other SH surface reported, it is easier to fabricate the particle/SR composites surfaces on a large scale with high efficiency and low cost. The mechanical robust superhydrophobicity of the particle/SR composites will be attractive for practical application for self-cleaning.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51263018), the S&T Supporting Plan of Jiangxi Province, Industrial Field (20133BBE50007) and the Education Bureau of Jiangxi Province, China (No. GJJ14631).References
- B. Bhushan and Y. C. Jung, Prog. Mater. Sci., 2011, 56, 1 CrossRef CAS.
- B. N. Sahoo and B. Kandasubramanian, RSC Adv., 2014, 4, 22053 RSC.
- J. Guo, S. Yu, J. Li and Z. G. Guo, Chem. Commun., 2015, 51, 6493 RSC.
- K. F. Babu and W. M. Choi, Compos. Sci. Technol., 2016, 122, 82 CrossRef CAS.
- J. Salabert, R. M. Sebastián and A. Vallribera, Chem. Commun., 2015, 51, 14251 RSC.
- R. J. Liao, Z. P. Zuo, C. Guo, A. Y. Zhuang, Y. Yuan, X. T. Zhao and Y. Y. Zhang, Cold Reg. Sci. Technol., 2015, 112, 87 CrossRef.
- L. H. Kong, X. H. Chen, L. G. Yu, Z. S. Wu and P. Y. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 2616 CAS.
- B. Wang, W. X. Liang, Z. G. Guo and W. M. Liu, Chem. Soc. Rev., 2015, 44, 336 RSC.
- D. M. Zang, R. W. Zhu, W. Zhang, J. Wu, X. Q. Yu and Y. F. Zhang, Corros. Sci., 2014, 83, 86 CrossRef CAS.
- Z. Z. Zhang, B. Ge, X. H. Men and Y. Li, Colloids Surf., A, 2016, 490, 182 CrossRef CAS.
- D. Mangini, C. Antonin, M. Marengo and A. Amirfazli, Cold Reg. Sci. Technol., 2015, 109, 53 CrossRef.
- Y. Y. Wang, M. Z. Li, T. Lv, Q. J. Wang, Q. M. Chen and J. F. Ding, J. Mater. Chem. A, 2015, 3, 4967 CAS.
- L. Y. Shen, B. L. Wang, J. L. Wang, J. H. Fu, C. Picart and J. Ji, ACS Appl. Mater. Interfaces, 2012, 4, 4476 CAS.
- A. Davis, Y. H. Yeong, A. Steele, I. S. Bayer and E. Loth, ACS Appl. Mater. Interfaces, 2014, 6, 9272 CAS.
- P. S. Brown and B. Bhushan, J. Colloid Interface Sci., 2015, 456, 210 CrossRef CAS PubMed.
- N. Wang, D. S. Xiong, Y. L. Deng, Y. Shi and K. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 6260 CAS.
- H. B. Wang, E. Chen, X. B. Jia, L. J. Liang and Q. Wang, Appl. Surf. Sci., 2015, 349, 724 CrossRef CAS.
- T. Rezayi and M. H. Entezari, Surf. Coat. Technol., 2015, 276, 557 CrossRef CAS.
- G. Momen, M. Farzaneh and R. Jafari, Appl. Surf. Sci., 2011, 257, 6489 CrossRef CAS.
- A. V. Raoa, S. S. Latthea, S. A. Mahadika and C. Kappenstein, Appl. Surf. Sci., 2011, 257, 5772 CrossRef.
- Y. H. Sung, Y. D. Kim, H. J. Choi, R. Shin, S. Kang and H. Lee, Appl. Surf. Sci., 2015, 349, 169 CrossRef CAS.
- Z. J. Liu, H. Y. Wang, E. Q. Wang, X. G. Zhang, R. X. Yuan and Y. J. Zhu, Polymer, 2016, 82, 105 CrossRef CAS.
- T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Adv. Mater., 2011, 23, 673 CrossRef CAS PubMed.
- Y. Li, S. S. Chen, M. C. Wu and J. Q. Sun, Adv. Mater., 2014, 26, 3344 CrossRef CAS PubMed.
- L. T. Yin, J. Yang, Y. C. Tang, L. Chen, C. Liu, H. Tang and C. S. Li, Appl. Surf. Sci., 2014, 316, 259 CrossRef CAS.
- F. Xue, D. M. Jia, Y. Li and X. L. Jing, J. Mater. Chem. A, 2015, 3, 13856 CAS.
- X. T. Zhu, Z. Z. Zhang, X. H. Men, J. Yang, K. Wang, X. H. Xu, X. Y. Zhou and Q. J. Xue, J. Mater. Chem., 2011, 21, 15793 RSC.
- C. H. Su, Y. Q. Xu, F. Gong, F. S. Wang and C. F. Li, Soft Matter, 2010, 6, 6068 RSC.
- S. A. Seyedmehdi, H. Zhang and J. Zhu, Appl. Surf. Sci., 2012, 258, 2972 CrossRef CAS.
- G. Momen and M. Farzaneh, Appl. Surf. Sci., 2012, 258, 5723 CrossRef CAS.
- S. A. Seyedmehdi, H. Zhang and J. Zhu, Prog. Org. Coat., 2016, 90, 291 CrossRef CAS.
- T. Smith, J. Colloid Interface Sci., 1980, 75, 51 CrossRef CAS.
- P. Liu, L. Cao, W. Zhao, Y. Xia, W. Huang and Z. L. Li, Appl. Surf. Sci., 2015, 324, 576 CrossRef CAS.
- H. Ogihara, J. Okagaki and T. Saji, Langmuir, 2011, 27, 9069 CrossRef CAS PubMed.
- T. Ishizaki and M. Sakamoto, Langmuir, 2011, 27, 2375 CrossRef CAS PubMed.
- C. Tan, Q. Li, P. Cai, N. Yang and Z. X. Xi, Appl. Surf. Sci., 2015, 328, 623 CrossRef CAS.
- X. Deng, L. Mammen, Y. F. Zhao, P. Lellig, K. Müllen, C. Li, H. J. Butt and D. Vollmer, Adv. Mater., 2011, 23, 2962 CrossRef CAS PubMed.
- G. Y. Wang, H. R. Wang and Z. G. Guo, Chem. Commun., 2013, 49, 7310 RSC.
- T. F. Wu, Y. Z. Pan and L. Li, Colloids Surf., A, 2011, 384, 47 CrossRef CAS.
- A. Das, H. T. Hayvaci, M. K. Tiwari, I. S. Bayer, D. Erricolo and C. M. Megaridis, J. Colloid Interface Sci., 2011, 353, 311 CrossRef CAS PubMed.
- L. Zhang, L. L. Li and Z. M. Dang, J. Colloid Interface Sci., 2016, 463, 266 CrossRef CAS PubMed.
- F. C. Yang, Y. Dong and Z. G. Guo, Colloids Surf., A, 2014, 463, 101 CrossRef CAS.
- Y. Lu, S. Sathasivam, J. Song, C. R. Crick, C. J. Carmalt and I. P. Parkin, Science, 2015, 347, 1132 CrossRef CAS PubMed.
- X. J. Liu, Y. Xu, K. Y. Ben, Z. Chen, Y. Wang and Z. S. Guan, Appl. Surf. Sci., 2015, 339, 94 CrossRef CAS.
- K. Seo, M. Y. Kim and D. H. Kim, Carbon, 2014, 68, 583 CrossRef CAS.
- A. Yildirim, T. Khudiyev, B. Daglar, H. Budunoglu, A. Okyay and M. Bayindir, ACS Appl. Mater. Interfaces, 2013, 5, 853 CAS.
- M. A. Nilsson, R. J. Daniello and J. P. Rothstei, J. Phys. D: Appl. Phys., 2010, 43, 045301 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04364j |
| This journal is © The Royal Society of Chemistry 2016 |


