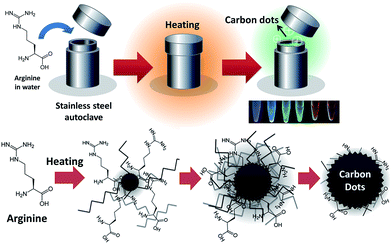
Pseudo-multicolor carbon dots emission and the dilution-induced reversible fluorescence shift†
Yu-Cheng Chena,
Cheng-Yung Niena,
Karunya Alberta,
Cheng-Che Wena,
You-Zung Hsieha and
Hsin-Yun Hsu*ab
aDepartment of Applied Chemistry, National Chiao Tung University, No. 1001 Ta-Hsueh Road, Hsinchu 30010, Taiwan. E-mail: hyhsu99@nctu.edu.tw
bInstitute of Molecular Science, National Chiao Tung University, No. 1001 Ta-Hsueh Road, Hsinchu 30010, Taiwan
First published on 27th April 2016
Abstract
Size- and color-tunable carbon dots (CDs) that emit blue to red using a single precursor in pure water were achieved by simple concentration and reaction time control. Distinct from conventional fluorophores, a reversible spectra shift of CD was observed upon dilution which could be a result of altered CD–CD inter-particle interaction, leading to minimized self-absorption.
Carbon dots (CDs) have been proposed to be a next-generation nanomaterial and may serve as a promising alternative for both in vitro and in vivo clinical applications including drug delivery, biosensing, and bioimaging due to their environmental friendliness, excellent biocompatibility, ease of preparation, and cost-effectiveness.1 They are luminescent and consist of an amorphous carbon or graphite structure with highly oxygenated groups at their surface. CDs have been synthesized via several approaches, such as laser ablation,2 electrooxidation of graphite,3 acid treatment of carbon precursors,4 hydrothermal synthesis,5 microwave-based pyrolysis,6 and room-temperature catalytic dehydration of small molecules.7 Although significant progress has been made, high-quality fluorescent CDs remain scarce. Their availability is limited by the following bottlenecks. First, most CDs possess weak fluorescence with low quantum yield (QY%) and are excited by UV light.8 Second, few carbon nanoparticles emit longer wavelengths (red to near-IR)9 and multiple precursors and additional extraction steps are necessary for preparation. Third, although some methods10 report milligram-scale quantities with 5–60% QY%, these methods require high-energy radiation-based synthesis followed by surface functionalization and purification, and simple, large-scale synthesis is usually difficult. Finally, although CDs that show excitation-dependent emission have been reported,9,11 such behavior was distinct from that of conventional fluorophores and the detailed mechanism by which CDs generate photoluminescence is not yet fully understood.12 Some studies have proposed that excitation-dependent emission bands might originate from the red-edge effect of inherited functionalities at the surface.13 The effect of band-to-band (π*–π) and interstate-to-band (π*–n) induced transitions leads to multicolor emissions. Amino functional groups might act as radiative recombination centers for tunable photoluminescent emission.14 Nevertheless, it remains difficult to determine general criteria for modulation of the fluorescent properties of CDs, as CDs synthesized from various precursors have usually been employed.
Compounds possessing amino or/and carboxyl groups have been proposed to be suitable candidates, as a result of their better inter-molecular interactions to initiate nuclei formation5,15 during CD synthesis. Amino acids possessing both amino and carboxyl groups are compatible with biological systems and several previous studies6,15,16 have reported the use of amino acid as ideal initial reactants. Herein, we demonstrated that multiple fluorescent CDs with significantly improved quantum yield could be synthesized using a single precursor in pure water, simply by tuning the concentration and the reaction time without necessity to employ additional catalysts or acid treatment. The high QY% (41.8%) of CD was synthesized which enabled in vivo bioimaging excited at 633 nm. In addition, our study revealed that the CD–CD inter-particle interaction could play a substantial role in the observed fluorescence, resulting in red-shifted “pseudo-multicolor” fluorescence. This strategy also enabled systematic investigation of the reaction parameters of CD synthesis, facilitating a better understanding of the observed photoluminescence of such materials.
The hydrothermal approach using a Teflon-lined autoclave reactor was employed due to its flexibility for easy scale-up and simple one-step CD synthesis (Scheme 1). Besides, under high temperature and pressure, the solubility of amino acid could be further improved. After an initial screen of 10 representative amino acids with characteristic side chains (Table S1†), the arginine (1 M) was selected as excellent CD precursor in this study according to the achieved highest QY% (compared with the other amino acids under identical hydrothermal conditions of 180 °C for 9 h). In the subsequent experiment to optimize the hydrothermal conditions, we tuned the concentration of the arginine precursor (1 M, 2 M, and 3 M (higher precursor concentrations were not examined owing to the arginine solubility)) and the reaction time (3 h, 6 h, 9 h, and 12 h). Carbon dots synthesized at specified precursor concentrations and times are abbreviated as CDconcentration,time. Six conditions for CD synthesis (CD1,3, CD1,6, CD1,9, CD1,12, CD2,9, and CD3,9) were compared and characterized by atomic force microscopy (AFM), Raman, Fourier-transform infrared (FT-IR), X-ray photoluminescence spectroscopy (XPS), and X-Ray Diffraction (XRD) (Table 1 and Fig. S1–S4†). The spherical CDs were well dispersed as observed in AFM images. Two characteristic bands, D and G, in Raman spectra of CDs were found in all samples and appeared featureless with increasing reaction time and concentration; this is probably due to the enhanced intrinsic photoluminescence background of CDs during synthesis. The FT-IR and deconvoluted high-resolution C 1s and N 1s XPS analysis confirmed the functional groups (enhanced amide I stretching and aldehyde C–H IR peaks, Fig. S2†) and chemical composition of the CDs (Fig. S3†). The obtained CDs presented a broad diffraction peak at 2θ = 20–22° in its XRD pattern (Fig. S4†), indicating that the interlayer spacing of the (002) diffraction peak is 0.392 nm, which was larger than that of graphite (0.34 nm). The increase in d value potentially revealed an increase in heterogeneous nature attributed to the introduction of more nitrogen-, and oxygen-containing groups.8,12 Improved crystallinity was observed in CD3,9 and this result was consistent with the found crystalline structures using high resolution TEM (Fig. S5†). The QY% was improved by the increased precursor content and the prolonged reaction time and it was inversely correlated with the chemical content (carbon (C)/nitrogen (N) + oxygen (O)) of the CDs (Table 1).
| XPS | Size (nm) | Quantum yield (%) | ||||
|---|---|---|---|---|---|---|
| C [%] | N [%] | O [%] | C/(N + O) | |||
| CD1,3 | 67.39 | 9.23 | 23.38 | 2.07 | 2.1 ± 0.8 | 14.6 |
| CD1,6 | 58.34 | 11.35 | 30.3 | 1.93 | 3.2 ± 1.3 | 27.9 |
| CD1,9 | 51.85 | 11.8 | 36.35 | 1.08 | 4.7 ± 1.2 | 32.6 |
| CD1,12 | 50.27 | 10.91 | 38.82 | 1.01 | 5.6 ± 1.3 | 34.5 |
| CD2,9 | 47.48 | 11.48 | 41.04 | 0.90 | 8.7 ± 3.7 | 38.4 |
| CD3,9 | 42.54 | 13.16 | 44.3 | 0.74 | 10.9 ± 3.4 | 41.8 |
The CD absorption spectra showed a broad shoulder with a maximum at 280 nm, which could be ascribed to the n–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band and the π–π* transition of the conjugated C
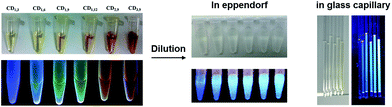
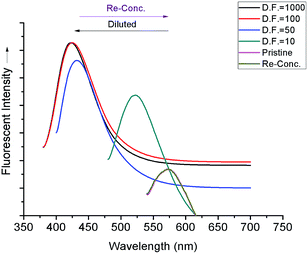
O band and the π–π* transition of the conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band (Fig. 1A). All synthesized CDs showed excitation-dependent fluorescence emission (Fig. S8†), and their λex and λem maxima were both red-shifted (from λex/λem = 380/440 nm for CD1,3, to λex/λem = 520/570 nm for CD3,9, Fig. 1A). We also observed red and near-IR emission of CDs, using lasers excited at 488 nm (Fig. S6†). Color changes from blue to red were observed under UV (365 nm) illumination (Fig. 2, pristine CDs). However, as shown in Fig. 1A, broadening of spectra and decreasing photoluminescence intensity at long wavelengths were observed. This decrease in intensity was inconsistent with the calculated QY% in Table 1. On the other hand, diluted CD suspensions (to make the absorbance consistent with 0.01 mg mL−1 quinine sulfate) revealed the increased fluorescence intensity exactly corresponding to QY% (Fig. 1B and 2, diluted CDs). The fluorescent emission of the CD samples was recovered after dilution. A full scan of both the excitation and the emission wavelengths (200–900 nm) of the other synthesized CDs photoluminescence confirmed this observation (Fig. S7 and S8†). Interestingly, we also found suspected upconversion photoluminescence after the dilution step (Fig. 1C), but nevertheless, additional investigation is undergoing to clarify such phenomenon as it could be resulted from the excitation of the short-wavelength second-order diffraction light coexisting with long-wavelength light in the spectrometer.17 We employed CD3,9 suspension to further investigate the dilution-induced fluorescence spectra changes. Reversible peak shifts were observed upon dilution and re-concentration (Fig. 3). Diluted CD3,9 revealed recovered blue emission whereas concentrated, pristine CD3,9 has shown quenched and red-shifted fluorescence. Such phenomena are distinct from those observed in conventional fluorescent molecules e.g. a high quinine sulfate concentration led to diminished fluorescence emission with only limited λem shifts (Fig. S9†). The synthesized CDs were additionally investigated in various solvents to examine their solubility, fluorescence spectra and the effects on QY% (Fig. S10 and Table S2†). All synthesized CDs were soluble in pure water and MeOH; with minute solubility in DMF and DMSO for CDs synthesized under high arginine concentration and longer reaction time, but were insoluble in THF and toluene (Fig. S10†). Superior QY% was found in pure water when comparing with that of the other tested solvents (Table S2†). The fluorescence spectra of all soluble CDs behaved similar excitation-dependent emission patterns (e.g. CD3,9 shown in Fig. S11†). However, due to the poor solubility in most organic solvents, the potential inner filter effect of CDs in high concentration was unavailable.
C band (Fig. 1A). All synthesized CDs showed excitation-dependent fluorescence emission (Fig. S8†), and their λex and λem maxima were both red-shifted (from λex/λem = 380/440 nm for CD1,3, to λex/λem = 520/570 nm for CD3,9, Fig. 1A). We also observed red and near-IR emission of CDs, using lasers excited at 488 nm (Fig. S6†). Color changes from blue to red were observed under UV (365 nm) illumination (Fig. 2, pristine CDs). However, as shown in Fig. 1A, broadening of spectra and decreasing photoluminescence intensity at long wavelengths were observed. This decrease in intensity was inconsistent with the calculated QY% in Table 1. On the other hand, diluted CD suspensions (to make the absorbance consistent with 0.01 mg mL−1 quinine sulfate) revealed the increased fluorescence intensity exactly corresponding to QY% (Fig. 1B and 2, diluted CDs). The fluorescent emission of the CD samples was recovered after dilution. A full scan of both the excitation and the emission wavelengths (200–900 nm) of the other synthesized CDs photoluminescence confirmed this observation (Fig. S7 and S8†). Interestingly, we also found suspected upconversion photoluminescence after the dilution step (Fig. 1C), but nevertheless, additional investigation is undergoing to clarify such phenomenon as it could be resulted from the excitation of the short-wavelength second-order diffraction light coexisting with long-wavelength light in the spectrometer.17 We employed CD3,9 suspension to further investigate the dilution-induced fluorescence spectra changes. Reversible peak shifts were observed upon dilution and re-concentration (Fig. 3). Diluted CD3,9 revealed recovered blue emission whereas concentrated, pristine CD3,9 has shown quenched and red-shifted fluorescence. Such phenomena are distinct from those observed in conventional fluorescent molecules e.g. a high quinine sulfate concentration led to diminished fluorescence emission with only limited λem shifts (Fig. S9†). The synthesized CDs were additionally investigated in various solvents to examine their solubility, fluorescence spectra and the effects on QY% (Fig. S10 and Table S2†). All synthesized CDs were soluble in pure water and MeOH; with minute solubility in DMF and DMSO for CDs synthesized under high arginine concentration and longer reaction time, but were insoluble in THF and toluene (Fig. S10†). Superior QY% was found in pure water when comparing with that of the other tested solvents (Table S2†). The fluorescence spectra of all soluble CDs behaved similar excitation-dependent emission patterns (e.g. CD3,9 shown in Fig. S11†). However, due to the poor solubility in most organic solvents, the potential inner filter effect of CDs in high concentration was unavailable.
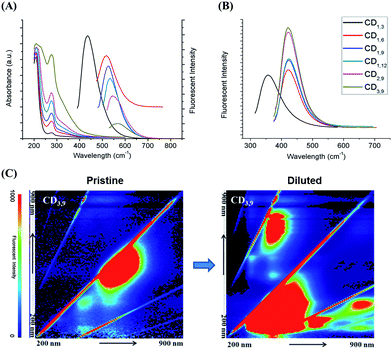 | ||
| Fig. 1 (A) The absorbance spectra and the fluorescent λem maxima of six synthesized CDs (excite at λex maxima, respectively); (B) normalized, maximal fluorescent emission peaks (CD samples diluted to the absorbance consistent with 0.01 mg mL−1 quinine sulfate). (C) The 3D fluorescence spectra of pristine CD3,9 (measured right after synthesis) and that of diluted CDs. Recovered intrinsic fluorescence of CD3,9 with enhanced fluorescence intensity was observed upon dilution. (x-axis: 200–900 nm of emission wavelengths; y-axis: 200–900 nm of excitation wavelengths; rainbow color gradient: florescence intensity; the HRTEM image of the CD3,9 is shown in Fig. S5†). | ||
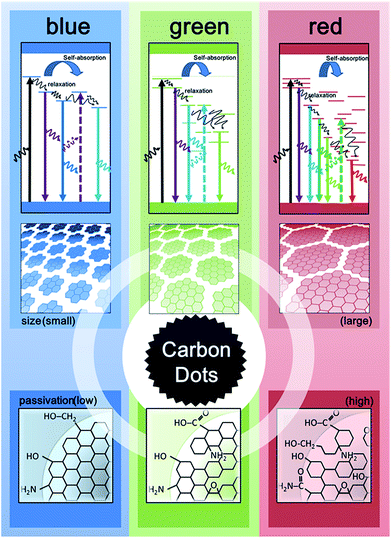
Developing high quality multicolor CDs for bioimaging is current research hotspot. As shown in our study, CDs with different particle sizes, chemical content/functional groups and QY% could be tuned by varying the precursor concentration and reaction time. An apparent red-shift of the fluorescence maximum was observed as reaction time increased from 3 h to 6 h. The high precursor concentration and a prolonged reaction time may facilitate carbonization to enable large particle growth, increasing the graphitic domains and the status of surface passivation. We also found although there were limited fluorescent λem maxima shifts after 6 h, significant improvement in hydrophilicity (due to the surface passivation) and QY% was achieved with prolonged reaction time (CD1,9 and CD1,12) and increasing arginine concentration (CD2,9 and CD3,9). We provide here a potential model (Fig. 4) to demonstrate the possibly involved mechanisms.18 High degree of surface passivation observed at a high arginine concentration using a long reaction time might result in the presence of surface energy traps that become emissive upon stabilization.19 Emission at longer wavelengths may have contributed to quantum-confinement20 and electronic conjugate structures, the larger sp2 nano-domain with appropriate size of band gaps.21 Additionally, the involvement of oxygen and nitrogen may change the sp2 domain by modulating the extent of the sp3 carbon matrix, creating defects that alter the distribution of emissive sites at the CD surface22 and energy levels corresponding to the maximum transition probability.23 As a result, different surface defects may generate different emissive sites, leading to excitation-dependent and tunable long-wavelength emission behaviors. Not only size but also surface status may have contributed to such alteration in the CD photoluminescent properties. Finally, the multiple emission centers may be present at CD surfaces, due to the heterogeneity (including size and passivation level) created during the hydrothermal process. Spectral distribution and increased non-radiative trapping24 may also occur. With concentrated CD suspensions (e.g. in the case of CD3,9), although only red-shifted spectra could be observed at longer wavelengths, blue-region emission was actually present but quenched (potentially caused by the inner filter effect25). When the energy gap is matched, the two individual emitters could undergo self-absorption or energy exchange with adjacent CDs, altering the fluorescence emission spectra.
In summary, we developed a single source, one-step method for size- and color-tunable CDs. This simple strategy improves and simplifies conventional processes for synthesis of CDs, which often involve strong acids or bases as catalysts and require multiple precursors for nucleation and passivation. By tuning the hydrothermal reaction time and the precursor concentration, the QY% of CDs could be significantly improved. Most importantly, we observed a dramatic spectral shift in diluted CDs, which is distinct from typical fluorophore–fluorophore interactions. We have shown that the CD–CD inter-particle interaction could play a substantial role in the observed fluorescence and the so-called “multicolor” fluorescence might actually originate from the inner filter effect, resulting in red-shifted fluorescence (but the inherent fluorescent is in fact located in blue region). As a result, the “pseudo-multicolor” CD emission was observed. Nonetheless, detailed investigations still will be required to clarify whether the source of such heterogeneity is the distribution of optical properties between CDs, demonstrating a collective response, or between individual emitters within CDs.
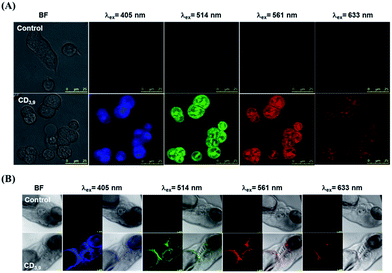
Additionally, our synthesized CDs showed low toxicity, wide-range pH stability (pH 3–11) and were resistant to photobleaching for several months under ambient conditions or continuous laser irradiation (18 W, 380–760 nm) (Fig. S12 and S13†). The excellent QY% (41.8%) of the CD3,9 in water solution has facilitated the development of new biocompatible optical tracers for real-time in vivo bioimaging (Fig. 5, S14 and S15†), enabling the excitation at longer wavelengths (633 nm) to minimize tissue damages. Intense excitation-dependent fluorescence signals could be observed throughout the zebrafish body, indicating that CDs had effectively entered the blood circulation. Such optical probe shows potential for further bio-distribution studies and drug delivery application. Finally, by taking advantage of dilution-induced fluorescence alteration resulted from CD–CD inter-particle interaction, the optical characteristics may be applied to swelling sensing of some hydrogel-based materials in the future.
Notes and references
- (a) Y.-C. Chen, C.-C. Wen, I. Liau, Y.-Z. Hsieh and H.-Y. Hsu, J. Mater. Chem. B, 2014, 2, 4100 RSC; (b) Y.-C. Chen, X.-C. Huang, Y.-L. Luo, Y.-C. Chang, Y.-Z. Hsieh and H.-Y. Hsu, Sci. Technol. Adv. Mater., 2013, 14, 044407 CrossRef; (c) C. B. Ma, Z. T. Zhu, H. X. Wang, X. Huang, X. Zhang, X. Qi, H. L. Zhang, Y. Zhu, X. Deng, Y. Peng, Y. Han and H. Zhang, Nanoscale, 2015, 7, 10162 RSC; (d) Y. Xu, M. Wu, Y. Liu, X. Z. Feng, X. B. Yin, X. W. He and Y. K. Zhang, Chem.–Eur. J., 2013, 19, 2276 CrossRef CAS PubMed.
- S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484 RSC.
- Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 5116 RSC.
- S. R. Zhang, Q. He, R. J. Li, Q. Wang, Z. Hu, X. Q. Liu and X. J. Chang, Mater. Lett., 2011, 65, 2371 CrossRef CAS.
- P. C. Hsu and H. T. Chang, Chem. Commun., 2012, 48, 3984 RSC.
- J. Jiang, Y. He, S. Li and H. Cui, Chem. Commun., 2012, 48, 9634 RSC.
- Y. Fang, S. Guo, D. Li, C. Zhu, W. Ren, S. Dong and E. Wang, ACS Nano, 2011, 6, 400 CrossRef PubMed.
- (a) S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546 CrossRef CAS; (b) S. Sahu, B. Behera, T. K. Maiti and S. Mohapatra, Chem. Commun., 2012, 48, 8835 RSC.
- S. K. Bhunia, A. Saha, A. R. Maity, S. C. Ray and N. R. Jana, Sci. Rep., 2013, 3, 1473 Search PubMed.
- X. Wang, L. Cao, S. T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y. P. Sun, Angew. Chem., Int. Ed., 2010, 49, 5310 CrossRef CAS PubMed.
- (a) J. Jeong, J. Jung, M. Choi, J. W. Kim, S. J. Chung, S. Lim, H. Lee and B. H. Chung, Adv. Mater., 2012, 24, 1999 CrossRef CAS PubMed; (b) L. Tang, R. Ji, X. Li, G. Bai, C. P. Liu, J. Hao, J. Lin, H. Jiang, K. S. Teng, Z. Yang and S. P. Lau, ACS Nano, 2014, 8, 6312 CrossRef CAS PubMed; (c) H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S.-T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430 CrossRef CAS PubMed.
- Y. Hao, Z. Gan, J. Xu, X. Wu and P. K. Chu, Appl. Surf. Sci., 2014, 311, 490 CrossRef CAS.
- (a) X. Gong, Q. Hu, M. C. Paau, Y. Zhang, S. Shuang, C. Dong and M. M. F. Choi, Nanoscale, 2014, 6, 8162 RSC; (b) S. K. Cushing, M. Li, F. Huang and N. Wu, ACS Nano, 2013, 8, 1002 CrossRef PubMed.
- G. Sandeep Kumar, R. Roy, D. Sen, U. K. Ghorai, R. Thapa, N. Mazumder, S. Saha and K. K. Chattopadhyay, Nanoscale, 2014, 6, 3384 RSC.
- A. Philippidis, D. Stefanakis, D. Anglos and D. Ghanotakis, J. Nanopart. Res., 2013, 15, 1414 CrossRef.
- S. Pei, J. Zhang, M. Gao, D. Wu, Y. Yang and R. Liu, J. Colloid Interface Sci., 2015, 439, 129 CrossRef CAS PubMed.
- J. Liu, X. L. Liu, H. J. Luo and Y. F. Gao, RSC Adv., 2014, 4, 7648 RSC.
- (a) Z. Gan, S. Xiong, X. Wu, T. Xu, X. Zhu, X. Gan, J. Guo, J. Shen, L. Sun and P. K. Chu, Adv. Opt. Mater., 2013, 1, 926 CrossRef; (b) C.-T. Chien, S.-S. Li, W.-J. Lai, Y.-C. Yeh, H.-A. Chen, I. S. Chen, L.-C. Chen, K.-H. Chen, T. Nemoto, S. Isoda, M. Chen, T. Fujita, G. Eda, H. Yamaguchi, M. Chhowalla and C.-W. Chen, Angew. Chem., Int. Ed., 2012, 51, 6662 CrossRef CAS PubMed; (c) M. J. Krysmann, A. Kelarakis, P. Dallas and E. P. Giannelis, J. Am. Chem. Soc., 2011, 134, 747 CrossRef PubMed.
- (a) Y.-P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S.-Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS PubMed; (b) L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y.-P. Sun, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS PubMed; (c) J. Zhou, C. Booker, R. Li, X. Zhou, T.-K. Sham, X. Sun and Z. Ding, J. Am. Chem. Soc., 2007, 129, 744 CrossRef CAS PubMed; (d) M. O. Dekaliuk, O. Viagin, Y. V. Malyukin and A. P. Demchenko, Phys. Chem. Chem. Phys., 2014, 16, 16075 RSC; (e) G. P. Lopinski, V. I. Merkulov and J. S. Lannin, Phys. Rev. Lett., 1998, 80, 4241 CrossRef CAS.
- W. L. Wilson, P. F. Szajowski and L. E. Brus, Science, 1993, 262, 1242 CAS.
- G. Eda, Y.-Y. Lin, C. Mattevi, H. Yamaguchi, H.-A. Chen, I. S. Chen, C.-W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505 CrossRef CAS PubMed.
- (a) F. Wang, S. Pang, L. Wang, Q. Li, M. Kreiter and C.-y. Liu, Chem. Mater., 2010, 22, 4528 CrossRef CAS; (b) L. Cao, M. J. Meziani, S. Sahu and Y.-P. Sun, Acc. Chem. Res., 2012, 46, 171 CrossRef PubMed.
- (a) D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS PubMed; (b) L. Bao, Z.-L. Zhang, Z.-Q. Tian, L. Zhang, C. Liu, Y. Lin, B. Qi and D.-W. Pang, Adv. Mater., 2011, 23, 5801 CrossRef CAS PubMed.
- X. Wen, P. Yu, Y.-R. Toh, Y.-C. Lee, A.-C. Hsu and J. Tang, Appl. Phys. Lett., 2012, 101, 163107 CrossRef.
- (a) X. Guo, C. F. Wang, Z. Y. Yu, L. Chen and S. Chen, Chem. Commun., 2012, 48, 2692 RSC; (b) F. Wang, M. Kreiter, B. He, S. Pang and C. Y. Liu, Chem. Commun., 2010, 46, 3309 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04309g |
| This journal is © The Royal Society of Chemistry 2016 |