Novel bodipy—cellulose nanohybrids for the production of singlet oxygen†
Abstract
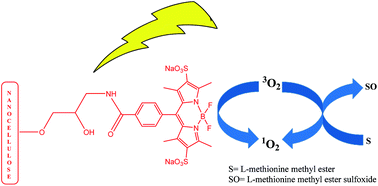
A novel hydrophilic carboxylated bodipy dye was synthesized and characterized by different spectroscopic techniques. The new nanomaterial was prepared by covalently conjugating it to amino nanocellulose, which was capable of producing singlet oxygen using a white light source.

 Please wait while we load your content...
Please wait while we load your content...