Janus Au–mesoporous silica nanocarriers for chemo-photothermal treatment of liver cancer cells
Zheng Wangab,
Yingshuai Wangac,
Mengmeng Lue,
Li Lia,
Yi Zhanga,
Xiao Zhengd,
Dan Shao*ade,
Jing Lid and
Wen-fei Dong*a
aCAS Key Laboratory of Bio-Medical Diagnostics, Suzhou Institute of Biomedical Engineering and Technology, Chinese Academy of Sciences, Suzhou 215163, China. E-mail: stanauagate@outlook.com; wenfeidong@126.com
bGraduate University of the Chinese Academy of Sciences, Beijing 100049, China
cState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, China
dDepartment of Pharmacology, Nanomedicine Engineering Laboratory of Jilin Province, College of Basic Medical Sciences, Jilin University, Changchun 130021, China
eDepartment of Biomedical Engineering, Columbia University, New York, NY 10027, USA
First published on 25th April 2016
Abstract
The combination of chemotherapy and photothermotherapy is emerging as a promising strategy for the treatment of liver cancer as a result of its synergistic efficacy. A safe and efficient drug-delivery system is highly desirable to ensure that the anticancer drug and photothermal agent can be simultaneously delivered to a tumor region to exert their synergistic effect with reduced side-effects. Uniform Janus Au–mesoporous silica nanoparticles with superior surface plasmon resonance properties and a high surface area were designed to integrate a high drug-loading capacity, pH-responsive properties, and a superior photothermal effect into a single carrier. The ability of the Janus nanoparticles loaded with doxorubicin to combine local specific chemotherapy with external near-infrared photothermotherapy significantly improved the therapeutic efficacy against liver cancer cells while exerting less toxicity on normal liver cells. Hence the reported doxorubicin-loaded Janus NPs may be promising therapeutic agents for efficacious and safe treatment of liver cancer.
1. Introduction
Liver cancer is the sixth most common cancer worldwide and the third most frequent cause of cancer death over last decade.1 Despite the development of standard strategies such as surgery, liver transplantation, chemotherapy, thermotherapy, and biotherapy, the overall survival of patients with liver cancer remains unsatisfactory as a result of the high rate of recurrence and metastasis.2 Chemotherapy is important in the clinical management of advanced liver cancer.3 However, a low therapeutic efficacy and severe side-effects limit its clinical applications as a result of drug resistance and non-selective cytotoxicity.4 Therefore successful treatment for liver cancer with improved therapeutic efficacy and reduced systemic toxicity requires the ability to combine chemotherapy with other treatment modalities. New combination treatments have been widely used in the clinic and have achieved immense popularity in the treatment of advanced liver cancer.5 Such strategies may not only achieve the synergistic effects of different treatment mechanisms to dramatically improve the overall therapeutic outcomes, but could also circumvent the drawbacks of a single therapeutic modality.6 Continuous efforts have led to the development of combined thermotherapy and chemotherapy for the treatment of liver cancer to augment the cytotoxicity of chemotherapeutic agents.7 Photothermotherapy (PTT) is a recently developed technique based on photothermal agents that strongly absorb near-infrared (NIR) light and convert it into cytotoxic heat to destroy tumor cells.8 PTT has gained in popularity because a specific amount of photoenergy is delivered directly into the tumor mass without causing systemic effects, thus making it a non-invasive, harmless, and highly efficient therapeutic technique.9 Chemotherapy and PPT have been reported to synergistically improve the therapeutic efficacy with reduced side-effects during liver cancer treatment.10 Nevertheless, to exert their maximum synergistic effect with minimal side-effects, accurate doses of the chemotherapeutic drug and photothermal agent should be simultaneously delivered to the same tumor cells after systemic administration.Nanoengineered drug-delivery systems have achieved great success in cancer research as a result of their improved therapeutic efficacy at the tumor site and reduced non-specific toxicity for vital functional organs.11–14 In this regard, Au nanoparticles (NPs) have been intensely investigated as a platform for PTT because their tunable surface plasmon resonance (SPR) matches the wavelength of the irradiated NIR light, while coherent oscillations of electrons in the conduction band induce photothermal conversion by converting the absorbed light to heat.15 To give the Au NPs synergistic properties, mesoporous silica has been used to protect the Au cores as a result of its unique properties, such as a high surface area, easy surface modification, and good biocompatibility, which give mesoporous silica-coated Au NPs the potential for use as an ideal carrier for loading with anti-tumor drugs in chemotherapy.16 Although core–shell Au–mesoporous silica NPs have superior applications in combined chemo-photothermal cancer treatment, the decreased anticancer drug-loading content and reduced Au absorption efficiency to NIR light might limit their clinical application.17 Therefore developing sophisticated nanocarriers with a better use of the intrinsic properties of both Au and mesoporous silica for chemo-PTT is critically important.
Janus NPs have attracted considerable attention because of their anisotropic surface properties and various functionalities that allow them to house several components for cancer diagnosis and treatment.18 Because of the combination of two distinct sides with different materials in one single unit and the asymmetry in surface chemistry, Janus NPs have more room to perform different functional roles in cancer treatment compared with traditional core–shell NPs.19 We have previously reported a facile and mild strategy to fabricate Au nanorod–mesoporous silica Janus NPs for use as multifunctional nanocarriers to achieve fluorescent imaging and PTT in liver cancer cells.20,21 However, to the best of our knowledge, the potential of these Janus Au–mesoporous silica NPs applied as multifunctional carriers for a synergistic treatment combining chemotherapy and PTT has not yet been explored.
We present here a modified sol–gel strategy to generate uniform Janus Au–mesoporous silica NPs with a small shift in the longitudinal SPR wavelength and a high surface area. The multifunctional NPs were designed to integrate a high drug-loading capacity, pH-responsive properties, and a superior photothermal effect into a single carrier. Our in vitro results established that Janus Au–mesoporous silica NPs can act as efficient carriers for the combined chemo-photothermal treatment of liver cancer and suggest their potential application in vivo.
2. Experimental section
2.1 Materials
Sodium borohydride, cetyltrimethyl ammonium bromide, tetraethyl orthosilicate, tetrachloroauric acid, fluorescein isothiocyanate (FITC), calcein acetoxymethyl ester (calcein AM, >90.0%), propidium iodide, Hoechst 33258, and sulforhodamine B (SRB) were obtained from Sigma-Aldrich (St Louis, MO, USA). Doxorubicin hydrochloride (DOX, >99%) was purchased from Shanghai Hualan Chemical Co. (Shanghai, China). RPMI-1640 medium was obtained from GIBCO. Fetal bovine serum (FBS), penicillin and streptomycin were from the Beyotime Institute of Biotechnology (Jiangsu, China). All reagents were commercially available products of analytical-grade purity and were used without further purification.2.2 Preparation of Janus NPs
Janus Au–mesoporous silica NPs with a controlled aspect ratio were synthesized using a modified sol–gel process according to a previously published method.20,21 Briefly, 50 mg of cetyltrimethyl ammonium bromide were dispersed in 10 mL of water and uniformly dispersed ultrasonically for at least 40 min until clarification; a solution of AuNPs was then added. The solution was poured into a three-necked flask and the temperature was maintained at 40 °C with stirring. Ammonia water (0.5 mL) was quickly added after 1 min and then a measured amount of tetraethyl orthosilicate was added dropwise into the reaction mixture for 30 min at a measured speed. The Janus NPs were obtained after quickly washing five times with ethanol (5000 rpm). To synthesize FITC-labeled Janus NPs, the silane coupling agent 3-aminopropyl-trimethoxysilane (APS) was covalently coupled to the amine-reactive dye FITC. FITC (0.5 mg) and APS (0.5 mL) were stirred in anhydrous ethanol (10 mL) in the dark at room temperature for 12 h and then 0.03 mL of the as-synthesized FITC–APS solution in ethanol was added to the reaction mixture to produce FITC-labeled Janus NPs using the same protocol.2.3 Characterization of Janus NPs
Transmission electron microscopy (TEM) images were obtained with a JEM-2100F transmission electron microscope (JEOL Ltd, Japan) with a 200 kV accelerating voltage. The surface area was determined by the Brunauer–Emmett–Teller method and the pore size distribution was calculated by the Barrett–Joyner–Halenda method. The UV-visible absorption spectra were measured on a U-3310 spectrophotometer (Hitachi, Japan). The temperature increase induced by NIR laser irradiation was measured by monitoring the temperature of DOX-loaded Janus NP dispersions in RPMI-1640 containing 10% FBS at various concentrations (0, 5, 10, 20, 40, and 80 μg mL−1) irradiated by a NIR laser (808 nm, 38 W cm−2). The temperature of the solution was measured every 10 s by a thermocouple microprobe submerged in the solution. The temperature of solutions of RPMI-1640 containing 10% FBS or pure water under radiation were measured as controls.2.4 DOX loading and release
To assess the pH-sensitive bonding ability of DOX, the surface of the Janus NPs was modified with carbonaceous structures using a previously published method.22 To study the loading behavior, 5 mg of Janus NPs were dispersed in 5 mL of DOX solution in PBS (0.5 mg mL−1). After stirring overnight, the DOX-loaded NPs were collected and washed three times with PBS. The DOX-loaded NPs were finally re-dispersed in PBS at a concentration of 10 mg mL−1. The amount of DOX adsorbed was determined by UV-visible spectrophotometry at 480 nm. To study the release behavior, 5 mg of DOX-loaded NPs were encapsulated into a dialysis bag (molecular weight cutoff 5000) and placed into 10 mL of PBS solutions with different pH values (pH 7.4 or 5.5). The release process was performed on a shaking table at 37 °C. The amount of released DOX in the supernatant was measured at timed intervals by UV-visible spectrophotometry.2.5 Cell uptake and intracellular drug release
The human hepatocellular carcinoma cell lines HepG2 and Bel-7402 and the human normal live cell line HL-7702 cells were seeded onto clean coverslips in a 24 well plate at a seeding density of 2 × 104 cells per well. To measure the intracellular localization of the Janus NPs, FITC-labeled Janus NPs (10 μg mL−1) were co-cultured with the cells for 3 h. The cells were then washed twice with chilled PBS and the nuclei were stained with Hoechst 33258 (5 mg mL−1) for 5 min. The locations of the NP were observed by confocal laser scanning microscopy (CLSM) using an Olympus FV1000 microscope equipped with a multi-line argon laser, 405 and 488 nm lasers, and a 30 mW laserclass 3D laser. The intracellular drug release of the DOX-loaded Janus NPs (10 μg mL−1) was measured using the same CLSM protocol.2.6 Assessment of cytotoxicity
The chemotherapy, PPT and combined chemo-photothermal effects on HepG2, Bel-7402, and HL-7702 cells were assessed using traditional SRB assays. The SRB assay is routinely used for the determination of cytotoxicity and is based on the measurement of the protein content of live cells.23 Cells were seeded into 96 well plates at a density of 5 × 103 cells per well overnight. The cells were then exposed to free Janus NPs, free DOX, and DOX-loaded Janus NPs at various concentrations (0.625, 1.25, 2.5, 5, 10, 20, and 40 μg mL−1) with or without NIR irradiation (38 W cm−2, 1–5 min) for 24 or 48 h. A 100 μL volume of 20% trichloroacetic acid was then added to the culture medium in each well and refrigerated at 4 °C for 3 h. The supernatant was then discarded and the plate was washed five times with water and air-dried. A 100 mL volume of 0.4% w/v SRB solution in 1% acetic acid was added to each well and incubated for 30 min at room temperature. Any unbound SRB was flicked off the plates and the plates were air-dried. The bound SRB was solubilized with 150 μL of 10 mM Tris–HCl added to each well and the plate was shaken for 5 min. The optical density at a wavelength of 570 nm was measured and the ratio of the cell viability relative to that of the control group was calculated from the SRB data based on three independent experiments.2.7 Live/dead assay
For the live/dead analysis, HepG2 or HL-7702 cells were seeded into a 24 well plate at a seeding density of 2 × 104 cells per well. After incubation overnight, the cells were exposed to free Janus NPs, free DOX, and DOX-loaded Janus NPs (10 μg mL−1) with or without NIR irradiation (38 W cm−2, 3 min) for 3 h. Each group of cells was washed with PBS, stained with calcein AM (2.0 μM) and propidium iodide (3.0 μM) and then observed using fluorescence microscopy. The live cells showed a green color and the dead cells showed a red color.2.8 Statistical analysis
Data were expressed as the mean ± SD values. The statistical significance of the data was determined by Student's t-test.3. Results and discussion
3.1 Preparation and characterization
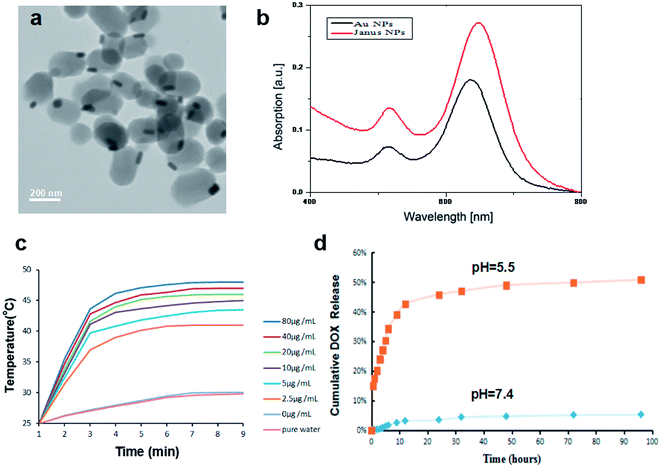
Janus Au–mesoporous silica NPs were prepared via a modified sol–gel method as reported previously and were characterized as shown in Fig. 1. The TEM image showed that the Janus NPs had a uniform rod-like structure consisting of an Au rod and a silica stick with dimensions of 40–60/10–20 nm and 200–250/100–120 nm, respectively (Fig. 1a). We studied the SPR properties of the Au NPs and Janus NPs via UV-visible spectrometry. Fig. 1b shows the characteristic transverse and longitudinal bands of the Au NPs at 520 and 640 nm; the Janus NPs had similar SPR properties with a slight red shift in the longitudinal SPR wavelength. Our previous work has demonstrated that the absorption offset of Janus NPs is much smaller than the 710 nm absorption offset of core–shell NPs with the same diameter silica rods.20 Therefore the strong NIR absorption of the Janus NPs suggested that these nanocarriers could be widely used in the photothermal treatment of tumors.To investigate the photothermal conversion efficiency of the Janus NPs, culture medium dispersions of Janus NPs with different concentrations (0–80 μg mL−1) were illuminated using an 808 nm laser at a power density of 38 W cm−2, chosen for its excellent penetration ability; the changes in temperature are shown in Fig. 1c. The temperature of pure water and the cell culture medium used as blanks only increased by 6 °C, whereas the temperature increase of the Janus NP solution followed a time- and concentration-dependent trend. At an NP concentration of 10 μg mL−1 with irradiation for less than 3 min, the temperature reached 43.0 °C, the critical temperature required to induce the death of cancer cells.
The surface area and pore volume were estimated to be 921.9 m2 g−1 and 0.62 cm3 g−1, respectively. The average pore diameter of 2.5 nm was measured by the Barrett–Joyner–Halenda method and indicated a porous structure, suggesting the possibility of drug loading. We introduced a carboxylate functional group onto the pore surface of the Janus NPs to achieve a responsive release in the low pH environment of cancer cells. Hydrophobic doxorubicin was chosen as a model drug because it is commonly used in the treatment of liver cancers.24 As determined by UV-visible spectrophotometry, the loading efficiency and drug-loading content of DOX in the Janus NPs were 73.9 and 25.2%, respectively. The drug-loading content of our Janus NPs is higher than that of the reported core–shell Au NPs, which might be a result of the large surface area of the Janus structure.25,26 The DOX release behavior from the DOX-loaded Janus NPs was investigated in PBS at different pH values (7.4 and 5.5). Fig. 1d shows that a slow release of DOX was observed at pH 7.4. Only 8% of the DOX was released after 96 h of dialysis. In contrast, a rapid release of DOX occurred at pH 5.5 and ca. 50% of the DOX was released after 96 h. This is because the DOX became more water-soluble at low pH as a result of the protonated amine group. This pH-dependent drug release of DOX-loaded Janus NPs is important in liver cancer chemotherapy because both the extracellular microenvironments of tumor tissues and the intracellular lysosomes and endosomes are acidic. The as-prepared Janus Au–mesoporous silica NPs showed a uniform morphology, high SPR, and mesoporous property, indicating that this nanocarrier might be beneficial in the treatment of liver cancer as a result of its superior photothermal conversion and pH-responsive drug-release capability.
3.2 Endocytosis and cytotoxicity
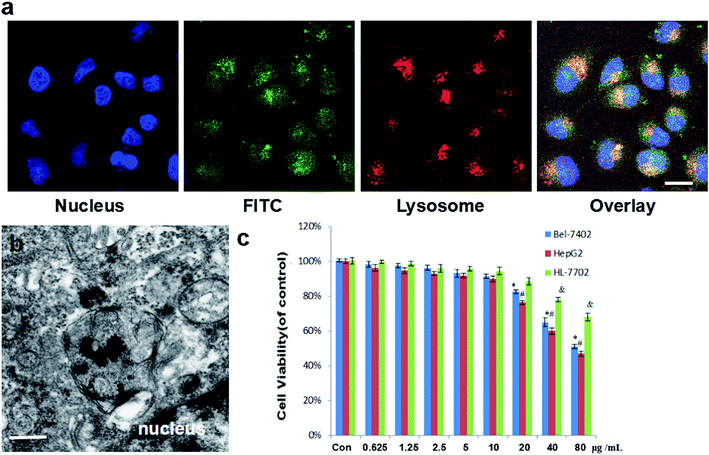
To explore the cellular uptake behavior of the Janus Au–mesoporous silica NPs in the human liver cancer HepG2 cells, the NPs were labeled using the fluorescence marker FITC and the subcellular localization of the FITC-labeled Janus NPs in HepG2 cells was investigated using CLSM. The overlay images in Fig. 2a show the co-localization of FITC-labeled Janus NPs with LysoTracker-Red in HepG2 cells after 3 h of incubation, indicating that some of the NPs entered the lysosomes, whereas others were present in the cytoplasm. These observations were further validated by Bio-TEM, in which the Janus NPs were found in endosomes distributed from the plasma membrane close to the nucleus (Fig. 2b). These results indicate that the Janus NPs have superior endocytotic properties and have the potential to release their payload into the cytoplasm of liver cancer cells. Endocytosis has a key role in cytotoxicity. The SRB assay was used to assess the cytotoxicity of the Janus NPs at different concentrations from 1.25 to 80 μg mL−1 on HepG2 and Bel-7402 liver cancer cell lines over 24 h. Fig. 2c shows that the Janus NPs had a concentration-dependent toxicity against both HepG2 and Bel-7402 cancer cells, whereas the cell viability significantly decreased to 90% when the concentration was >10 μg mL−1. The cytotoxicity effect of the Janus NPs in liver cancer cells was stronger than in human normal liver cancer HL-7702 cells, which is similar to the specific manner of killing quantum dot–lipid complexes toward cancer cells reported previously.21 Considering both the better photothermal conversion ability and lower cytotoxicity, we chose 10 μg mL−1 as the optimum dose for this drug-delivery carrier for the treatment of liver cancer cells.3.3 Intracellular drug release and anticancer effect
To further determine the pH-dependent drug-releasing properties of DOX-loaded NPs in vitro, we investigated their intracellular drug-release behavior in HepG2 cells using CLSM. Fig. 3 shows that more red fluorescent signals from DOX were found in HepG2 cells after 3 h of incubation with DOX-loaded Janus NPs than in cells incubated with free DOX. Only the free DOX in the cytoplasm can enter the cell nucleus and insert into DNA to cause cell death; DOX-loaded Janus NPs are effective carriers and can enter liver cancer cells to release DOX into the cytoplasm, exerting a similar anti-tumor effect to that of free DOX.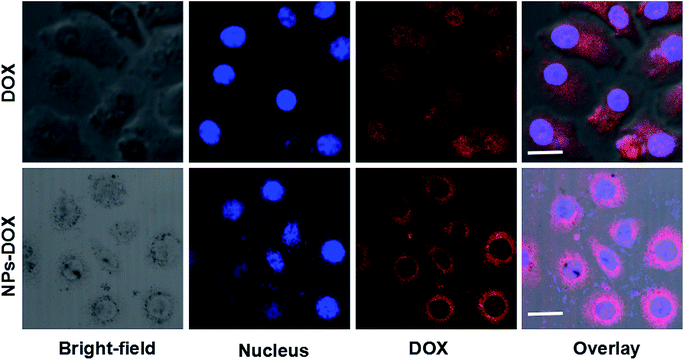 | ||
| Fig. 3 CLSM images of HepG2 cells treated with DOX-loaded Janus NPs for 3 h. The cell nuclei were stained with Hoechst 33258 (blue); scale bars = 10 μm. | ||
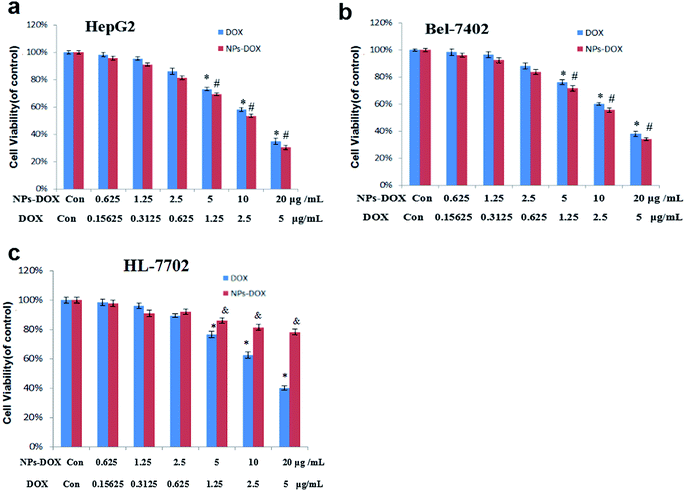
HepG2, Bel-7402 and HL-7702 cells were used to evaluate the in vitro anticancer efficacy of DOX-loaded Janus NPs through the SRB assay. In terms of their cytotoxicity toward HepG2 cells, both the DOX-loaded Janus NPs and an equal dose of free DOX showed comparable cytotoxicity after a 24 h incubation (Fig. 4a). This was also demonstrated with the Bel-7402 cells (Fig. 4b). Normal HL-7702 cells showed significant insensitivity to DOX-loaded Janus NPs compared with free DOX in 24 h culture (Fig. 4c), suggesting that pH-sensitive releasing of the drug plays a key part in the difference in cytotoxicity of DOX-loaded Janus NPs. These results demonstrate that the anticancer properties of DOX-loaded Janus NPs originates from the superior capacity for endocytosis and the pH-sensitive drug release in cancer cells. They could be applied as multifunctional carriers for combined chemo-photothermal treatment in liver cancer cells.
3.4 In vitro chemo-photothermal treatment
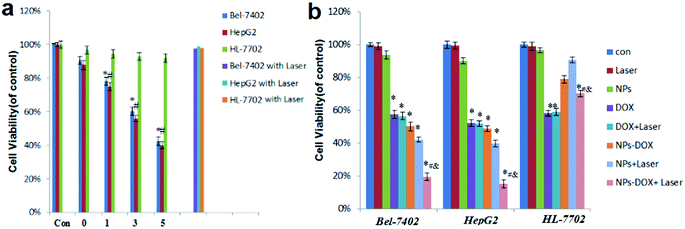
To study the localized photothermal effect in vitro, HepG2, Bel-7402, and HL-7702 cells were exposed to a laser at a power density of 38 W cm−2 for 0, 1, 3, or 5 min immediately after the addition of a blank Janus NP dispersion (10 μg mL−1) or in the absence of the NPs, followed by an SRB assay to determine the cell viability. Fig. 5a shows that, compared with the non-irradiated group, the viability of HepG2 and Bel-7402 cells was reduced by <5%, even after 5 min of irradiation. In contrast, when pre-treated with Janus NPs, the irradiation caused a time-dependent toxicity effect on the cell viability. After 3 min of irradiation, about 40% of the liver cancer cells were destroyed as a result of heat conduction at 24 h, whereas over 95% of the normal liver cells survived because cancer cells have a low heat tolerance compared with normal cells. These results suggest that the Janus NPs have a significant PTT effect on tumor cells at low laser power densities and short irradiation times without damaging normal cells and therefore could act as an effective and safe treatment for liver cancer.On the basis of these observations, we further evaluated the chemo-photothermal cytotoxicity of DOX-loaded Janus NPs on HepG2 or Bel-7402 cells through the SRB assay (Fig. 5b). The group of HepG2 or Bel-7402 cells alone and the group of the same cells under laser irradiation showed no significant cell death with 10 μg mL−1 of Janus NPs. The group of cells incubated with DOX-loaded Janus NPs or an equal dose of free DOX, and the group of cells incubated with blank Janus NPs with 3 min of laser irradiation showed moderate cell death of almost half of the cells. The group of liver cancer cells incubated with DOX-loaded Janus NPs with 3 min of laser irradiation showed a much higher cell death compared with the other groups, but had no apparent effect on the normal liver cells. This synergistic effect is probably ascribed to the increased temperatures enhancing the heat sensitivity of the cancer cells exposed to DOX.
The live/dead assay was used to evaluate the efficacy of the chemo-photothermal treatment. As shown in Fig. 6 and 7, the treatment with DOX-loaded Janus NPs under 3 min of laser irradiation resulted in almost complete cell death in the HepG2 cells, which showed more red fluorescence than the other groups, which was in line with the cell viability results. Compared with chemotherapy or PTT alone, these results showed that chemo-photothermal treatment with DOX-loaded Janus NPs had a synergistically higher therapeutic efficacy on liver cancer cells than on normal liver cells. This study provides an efficient and safe nanocarrier approach for the combination treatment of liver cancer.
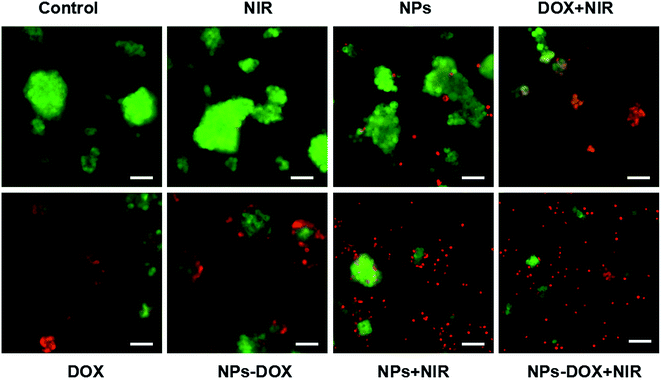 | ||
| Fig. 6 Fluorescence images of calcein AM/propidium iodide co-stained HepG2 cells; scale bar = 50 μm. | ||
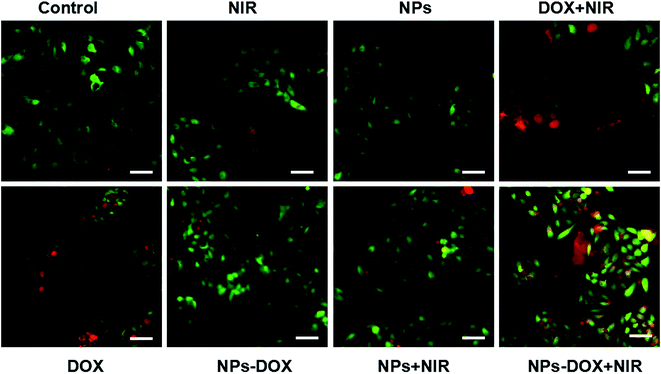 | ||
| Fig. 7 Fluorescence images of calcein AM/propidium iodide co-stained HL-7702 cells; scale bars = 50 μm. | ||
4. Conclusions
We successfully prepared uniform Janus Au–mesoporous silica NPs with a superior SPR wavelength and high surface area. The obtained DOX-loaded Janus NPs not only had a high drug-loading capacity, but also pH-responsive drug-release properties. Compared with chemotherapy or PTT alone, the DOX-loaded Janus NP showed higher toxicity to liver cancer cells than normal liver cells on irradiation with 808 nm NIR light, indicating the effectiveness and safety of the combined chemo-photothermal treatment. Our work highlights the potential of Janus Au–mesoporous silica NPs as nanocarriers to improve the efficacy of the treatment of liver cancer.Conflicts of interest
There is no conflict of interest in this manuscript.Acknowledgements
The work was supported by the Science and Technology Department of Suzhou City (No. ZXY201434).Notes and references
- M. Maluccio and A. Covey, Ca-Cancer J. Clin., 2012, 62, 394–399 CrossRef PubMed.
- J. Bruix, G. J. Gores and V. Mazzaferro, Gut, 2014, 63, 844–855 CrossRef CAS PubMed.
- V. W. Lam, C. Spiro, J. M. Laurence, E. Johnston, M. J. Hollands, H. C. Pleass and A. J. Richardson, Ann. Surg. Oncol., 2012, 19, 1292–1301 CrossRef PubMed.
- R. G. Gish, C. Porta, L. Lazar, P. Ruff, R. Feld, A. Croitoru, L. Feun, K. Jeziorski, J. Leighton, J. Gallo and G. T. Kennealey, J. Clin. Oncol., 2007, 25, 3069–3075 CrossRef CAS PubMed.
- R. Lencioni and L. Crocetti, Image-Guided Cancer Therapy: A Multidisciplinary Approach, 2013, pp. 339–343 Search PubMed.
- J. J. Knox, S. P. Cleary and L. A. Dawson, J. Clin. Oncol., 2015, 33, 1835–1844 CrossRef CAS PubMed.
- K. F. Chu and D. E. Dupuy, Nat. Rev. Cancer, 2014, 14, 199–208 CrossRef CAS PubMed.
- Z. Zhang, J. Wang and C. Chen, Adv. Mater., 2013, 25, 3869–3880 CrossRef CAS PubMed.
- H. Yu, Z. Cui, P. Yu, C. Guo, B. Feng, T. Jiang, S. Wang, Q. Yin, D. Zhong and X. Yang, Adv. Funct. Mater., 2015, 25, 2489–2500 CrossRef CAS.
- L. Wu, M. Wu, Y. Zeng, D. Zhang, A. Zheng, X. Liu and J. Liu, Nanotechnology, 2015, 26, 025102 CrossRef CAS PubMed.
- F. Kratz and A. Warnecke, J. Controlled Release, 2012, 164, 221–235 CrossRef CAS PubMed.
- D. Shao, J. Li, Y. Pan, X. Zhang, X. Zheng, Z. Wang, M. Zhang, H. Zhang and L. Chen, Biomater. Sci., 2015, 3, 833–841 RSC.
- D. Shao, J. Li, F. Guan, Y. Pan, X. Xiao, M. Zhang, H. Zhang and L. Chen, Int. J. Nanomed., 2014, 9, 5753–5769 CrossRef CAS PubMed.
- G. J. Xu, S. J. Liu, H. Niu, W. P. Lv and R. A. Wu, RSC Adv., 2014, 4, 33986–33997 RSC.
- A. J. Mieszawska, W. J. Mulder, Z. A. Fayad and D. P. Cormode, Mol. Pharm., 2013, 10, 831–847 CrossRef CAS PubMed.
- Z. Zhang, L. Wang, J. Wang, X. Jiang, X. Li, Z. Hu, Y. Ji, X. Wu and C. Chen, Adv. Mater., 2012, 24, 1418–1423 CrossRef CAS PubMed.
- W. Li and D. Zhao, Adv. Mater., 2013, 25, 142–149 CrossRef CAS PubMed.
- L. T. Tran, S. Lesieur and V. Faivre, Expert Opin. Drug Delivery, 2014, 11, 1061–1074 CrossRef CAS PubMed.
- L. Zhang, Y. Chen, Z. Li, L. Li, P. Saint-Cricq, C. Li, J. Lin, C. Wang, Z. Su and J. I. Zink, Angew. Chem., Int. Ed., 2016, 127, 1–5 Search PubMed.
- Y.-S. Wang, D. Shao, L. Zhang, X.-L. Zhang, J. Li, J. Feng, H. Xia, Q.-S. Huo, W.-F. Dong and H.-B. Sun, Appl. Phys. Lett., 2015, 106, 173705 CrossRef.
- G. Liu, Q. Li, W. Ni, N. Zhang, X. Zheng, Y. Wang, D. Shao and G. Tai, Int. J. Nanomed., 2015, 10, 6075 Search PubMed.
- D. Shao, Z. Wang, W. F. Dong, X. Zhang, X. Zheng, X. X. Xiao, Y. S. Wang, X. Zhao, M. Zhang and J. Li, Chem. Biol. Drug Des., 2015, 86, 1548–1553 CAS.
- V. Vichai and K. Kirtikara, Nat. Protoc., 2006, 1, 1112–1116 CrossRef CAS PubMed.
- H. J. Prajapati, R. Dhanasekaran, B. F. El-Rayes, J. S. Kauh, S. K. Maithel, Z. Chen and H. S. Kim, J. Vasc. Intervent. Radiol., 2013, 24, 307–315 CrossRef PubMed.
- Z. J. Zhang, L. M. Wang, J. Wang, X. M. Jiang, H. H. Li, Z. J. Hu, Y. L. Ji, X. C. Wu and C. Y. Chen, Adv. Mater., 2012, 24, 1418–1423 CrossRef CAS PubMed.
- J. Liu, C. Detrembleur, M. C. De Pauw-Gillet, S. Mornet, C. Jérôme and E. Duguet, Small, 2015, 1, 2323–2332 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |