DOI:
10.1039/C6RA04172H
(Paper)
RSC Adv., 2016,
6, 44655-44667
Removal of endocrine disruptor di-(2-ethylhexyl)phthalate by modified polythiophene-coated magnetic nanoparticles: characterization, adsorption isotherm, kinetic study, thermodynamics†
Received
16th February 2016
, Accepted 15th April 2016
First published on 18th April 2016
Abstract
Core–shell magnetic nanoparticles have received significant attention and are actively explored due to their prospective applications. In the current study, superparamagnetic nanosorbent poly(phenyl(4-(6-thiophen-3-yl-hexyloxy)-benzylidene)-amine)/Fe3O4 nanoparticles (Fe3O4@P3TArH) was successfully synthesized via a simplistic method for the enhanced extraction of a potent endocrine disruptor, di-(2-ethylhexyl)phthalate (DEHP). The synthesized materials were characterized by Fourier transform infra-red (FTIR), X-ray diffractometry (XRD), Brunauer–Emmett–Teller (BET) surface area analysis, field emission scanning electron microscope (FESEM), transmission electron microscopy (TEM), and vibrating sample magnetometer (VSM). The extraction efficiencies of the synthesized sorbent materials were evaluated by monitoring the extraction of DEHP from aqueous solution. Removal of DEHP using Fe3O4@P3TArh was found to be pH and temperature dependent with a maximum adsorption capacity found to be at 298.15 K at pH 7 and the adsorption kinetics followed a pseudo second-order kinetics model. Thermodynamic studies revealed that adsorption occurred heterogeneously on the adsorption sites, and adsorption of di-(2-ethylhexyl)phthalate onto Fe3O4@P3TArh was found to be spontaneous, feasible, ordered, and exothermic. The activation energy was determined to be −40.6 kJ mol−1, which indicated the adsorption process was physisorption.
1. Introduction
Contamination caused by phthalates in the form of plasticizers for polyvinyl chloride resins, adhesives, and cellulose film coatings has recently increased due to widespread use.1 They are favored as plasticizing agents due to their capabilities of enhancing the placidity and the flexibility of plastics.2,3 To date, 93% of plasticizers are phthalates, while the rest are esters and polyesters of adipate, phosphoric acid, and sebacic acid.4 Due to massive consumption of phthalates by many industries, these toxic materials eventually penetrate into the environment. Di-(2-ethylhexyl)phthalate (DEHP) is an important molecule in the phthalates family which is extensively used in medical devices, children's toys, water containers, textiles, and all kinds of packaging.5–8 It is harmful to humans, especially children, due to the fact that there is no chemical linkage between DEHP molecules and the plastic-based compound, making them easily released to the environment where they can come into contact with consumers.9 DEHP is also classified as an endocrine disruptor in the context of reproductive health.10–12 Moreover, DEHP is listed as a significant pollutant in many countries due to its carcinogenic nature.13
Due to its toxic and carcinogenic nature, removal of DEHP from waste waters recently has gained immense scientific interest with many crucial methods being reported by several researchers for its removal from an aqueous environment. Biodegradation of phthalates using bacteria culture is one of the potential techniques and has been reported by many researchers.14,15 However, this method is time consuming, and does not result in complete degradation of phthalates. The coagulation step, during the water treatment process, is useful for removing organic pollutants. Nevertheless, degrading phthalates via coagulation using ferric chloride seemed ineffective.16,17 Another method, called the advanced oxidation process (AOP), is based on addition of a highly active oxidizing agent which proficiently oxidizes very stable molecules, such as the degradation of DEHP via UV/H2O2.18 Despite the fact that the AOP technique could result in significant degradation, this method requires expensive and toxic reagents, which themselves are considered as pollutants, and are difficult to handle. The adsorption method has been proved to be an effective technique with higher efficacy and capability to remove pollutants on a large scale besides having other advantages including recovery and recycling of adsorbents. Thus, adsorption would be a competent technique for the efficient removal of larger quantities of phthalates from polluted wastewater.19
Fe3O4 magnetic nanoparticles are one of the most important nanomaterials and technologically important objects for physical and chemical research, with many promising applications. Magnetic nanoparticles (MNPs) were demonstrated to be an efficient adsorbent for adsorption of trace amounts of organic and inorganic analytes from complex media, since they do not require any centrifugations or filtrations.20 Use of MNPs coated with polymeric material gained much interest, as it enhances surface functionalization and protects the magnetic core from environmental agitation. Moreover, the presence of polymeric surface coated magnetic nanoparticles prevented aggregation and homogenized the nanoparticles core shell distribution within the suspension media.21 Elevating research interest on using conducting polymer as a polymeric coating material is due to its multifunctional and diverse properties, which includes its cheaper costs, environmental stability, and mechanical robustness.22–24
Herein, we report a facile synthesis route to develop a newly designed magnetic nanoparticle of Fe3O4 coated with polythiophenes containing Schiff-base, biphenyl pendant, and aliphatic linkage, with its adsorptive performance linked to the removal of DEHP from aqueous solutions. The presence of aliphatic and aromatic side groups in the material significantly enhanced the adsorptive performance for the removal of DEHP from aqueous solution in terms of hydrophobic and π–π interactions. Oxidation/corrosion may reduce magnetic moment of Fe3O4 which is responsible for diminishing its magnetization and therefore became a limiting factor in efficient utilization of Fe3O4 in various applications. To the best of our knowledge, some Schiff bases containing thiophene substituents have been previously reported as effective erosion inhibitors.25–27 In the present investigation, Schiff base groups were designed for shielding the magnetic core of Fe3O4 from corrosion besides augmenting the adsorption of phthalates due to physical interactions. Simplistic synthesis, cost-effectiveness, and enhanced adsorption behaviour towards DEHP make these nanosorbents a potential adsorbent material for phthalates waste water treatment.
2. Materials and methods
2.1 Reagent and chemicals
Analytical grade ferric chloride, ferrous chloride, ammonia solution (25 wt%), thiophene, 4-hydroxybenzaldehyde, acetonitrile, potassium permanganate, 4-aminophenol, 3-bromothiophene, 1,6-dibromohexane, N-bromosuccinimide, acetic acid, sodium hydrogen bicarbonate, potassium iodide, potassium carbonate, tetrahydrofuran, methanol, hydrochloric acid, acetone, and ethyl acetate were purchased from Merck (Darmstadt, Germany). Acetone was procured from Fisher Scientific (Loughborough, UK). Thiophene carboxaldehyde, polyvinyl alcohol, and n-butyllithium (2.0 M in cyclohexane) were obtained from Sigma-Aldrich (Milwaukee, WI, USA). Magnesium sulfate anhydrous, ethanol denatured, and hexane were received from J. Kollins (Parkwood, Australia), while dimethyl sulfoxide-d6 (DMSO-d6) and di-(2-ethylhexyl)phthalate (DEHP) were purchased from Acros Organics (Geel, Belgium). Ultrapure water was prepared with a model Aqua Max-Ultra ultra-pure water purification system (Zef Scientific Inc., San Diego, CA, USA). Stock solutions of 1000 mg L−1 of standards were prepared by dissolving appropriate amounts of compounds in methanol, which remains stable for three months if stored in a refrigerator at 4 °C. Working standard solutions were prepared daily by diluting the stock standard solution to the required concentrations.
2.2 Synthesis method
2.2.1 Preparation of (phenyl-(4-(6-thiophen-3-yl-hexyloxy)-benzylidine)-amine) (3TArH). A mixture of 4-((phenylimino)methyl)phenol (1.97 g, 10 mmol) (S1) anhydrous potassium carbonate (4.14 g, 30 mmol) (S2), and 18-crown-6 ether (16.6 mg, 0.1 mmol) was stirred in dry and degassed acetone (50 mL) at room temperature followed by the addition of 3-(6-bromohexylthiophene) (0.81 g, 2 mmol) (Scheme 1). The reaction mixture was refluxed under nitrogen with stirring for 24 h. Later, the reaction mixture was cooled to room temperature and poured into the saturated solution of potassium carbonate. The organic phase was collected and washed with water (100 mL), dried with anhydrous sodium sulfate, and subsequently filtered. The solvent was removed via reduced pressure, and the residue was dried under vacuum to produce a crude product.28 Purification was accomplished by column chromatography using silica, with 25% hexane in chloroform to afford the monomer.
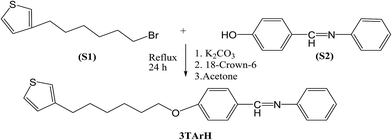 |
| | Scheme 1 Synthesis of 3TArH. | |
2.2.2 Preparation of modified polythiophene-coated magnetic nanoparticles (Fe3O4@P3TArH NP) and polythiophene coated magnetic nanoparticles (Fe3O4@PTh). Fe3O4@P3TArH NPs were synthesized in two steps. Briefly, Fe3O4 was prepared by a co-precipitation method.29 FeCl3·6H2O (8.48 g) and FeCl2·4H2O (2.25 g) were dissolved in 400 mL deionized water under nitrogen atmosphere via vigorous stirring (1000 rpm) at 80 °C. Then, a 20 mL ammonia solution (25% wt) was added to the solution. The color of the bulk solution immediately changed from orange to black. After stirring the mixture for 5 min, the Fe3O4 NPs precipitates were obtained via magnetic decantation, and washed several times with deionized water. Finally, the Fe3O4 NPs were dried in a vacuum oven at 70 °C for 12 h. Next, the surface of the Fe3O4 NPs was modified by coating with newly designed modified thiophene monomers containing biphenyl pendant (3TArH) via oxidation polymerization with the generation of ferric cations on Fe3O4 NPs surface.21 Fe3O4 NPs (1 mmol, 0.236 g) was discretized in a PVA aqueous solution (0.5 M). Later, 3TArH (10 mmol, 3.640 g) was added into the mixed solution via vigorous stirring. Subsequently, 30 mL of HCl (0.5 M) solution was introduced into the mixture. Then, the product was dried under vacuum at 70 °C for 12 h.Additionally, the above experiments were repeated using freshly distilled thiophene monomer (10 mmol, 0.84 g) to obtain polythiophene coated magnetic nanoparticles (Fe3O4@PTh) which acted as a control adsorbent for comparative study of the adsorption efficiency of different adsorbents.
2.3 Characterization of the samples
Fourier transform infrared (FT-IR) spectra were recorded on a PerkinElmer FT-IR between 4000 and 400 cm−1 (PerkinElmer, Massachusetts, USA). Structural elucidation was determined using 1H NMR, JEOL 400 MHz (JEOL, Tokyo Japan). X-ray powder diffraction (XRD) analysis was conducted with Panalytical model Empyrean at 40 kV and 35 mA using Cu Kα radiation (λ = 1.54059 Å) (Panalytical, Almelo, Netherlands). Morphological analysis of the synthesized products were performed using a JEOL JSM-7600F field emission scanning electron microscope operated at 3 kV (JEOL, Tokyo Japan) and transmission electron microscopy (TEM) analysis using an FEI Tecnai G2 spectra microscope (FEI, Hillsboro, USA). The magnetic property was tested using a vibration sample magnetometer (VSM) Model 9600 (Quantum Design Inc., San Diego, USA). Magnetization measurements were carried out in an external field of up to 15 kOe at room temperature. The thermal stability of Fe3O4@P3TArH was investigated by thermogravimetric analysis (TGA); model TGA-STA 1500, with a heating rate of 10 °C min−1 between 25 and 900 °C under nitrogen atmosphere (PerkinElmer, Massachusetts, USA). Brunauer–Emmett–Teller (BET) analysis was carried out using a Micromeritics ASAP2020 surface area analyser for determining the pore diameter and the specific surface area of nanosorbents (Micromeritics, Georgia, USA).
2.4 Batch experiments
Experimental parameters were optimized using batch experiments for type of adsorbent, effect of pH, kinetics and thermodynamic studies, effect of initial concentration, equilibrium studies, and reusability studies. Sorption experiments were determined by the following batch method: in each experiment, 5 mg of adsorbent was mixed with 5 mL of an aqueous solution of (DEHP) at a known concentration in a tightly sealed vial. The solution was shaken for 1 h on a shaker at room temperature. After the adsorption process, the adsorbent was separated by magnetic decantation, and the residual concentration was determined using a Shimadzu Ultraviolet-Visible spectrophotometer (UV-Vis), equipped with 1 cm quartz cells (Shimadzu, Kyoto, Japan). All the samples were performed in triplicate. The removal efficiency, R (%) was calculated using the following equation:| |
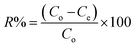 | (1) |
The amount of DEHP adsorbed per unit mass of the adsorbent (qe) was calculated as:
| |
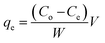 | (2) |
Co and
Ce are the initial and equilibrium concentrations of the solutions (mg L
−1), respectively.
V (L) is the volume of the solution, and
W (g) is the mass of the dry adsorbent being used.
3. Result and discussion
3.1 Characterization of the nanocomposites
3.1.1 FT-IR analysis. Fig. 1 show several additional peaks in the spectrum of Fe3O4@P3TArH, proportional to the Fe3O4 NPs spectrum, which belong to the coating. The intense peaks in the range of ∼3400 cm−1 for Fe3O4 and Fe3O4@P3TArH indicated the presence of OH vibration. For both P3TArH and Fe3O4@P3TArH, the C–H aromatic stretching peaks were observed at 2980 cm−1, whereas C–H sp3 stretching (hexyl aliphatic side) occurred at 2934 cm−1. Meanwhile, Schiff base (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) was observed at 1601 cm−1 for P3TArH, and 1685 cm−1 for the Fe3O4@P3TArH.30 C
N) was observed at 1601 cm−1 for P3TArH, and 1685 cm−1 for the Fe3O4@P3TArH.30 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic stretching peaks exhibited at 1562 cm−1 and 1462 cm−1 for P3TArH, whereas 1564 cm−1 and 1462 cm−1 for Fe3O4@P3TArH. Two absorption band peaks at 1250 and 1072 cm−1 indicated the presence of C–O observed in the P3TArH as well as Fe3O4@P3TArH. The strong absorption peaks at 868 cm−1 for P3TArH and 846 cm−1 for the nanocomposite indicated the presence of C
C aromatic stretching peaks exhibited at 1562 cm−1 and 1462 cm−1 for P3TArH, whereas 1564 cm−1 and 1462 cm−1 for Fe3O4@P3TArH. Two absorption band peaks at 1250 and 1072 cm−1 indicated the presence of C–O observed in the P3TArH as well as Fe3O4@P3TArH. The strong absorption peaks at 868 cm−1 for P3TArH and 846 cm−1 for the nanocomposite indicated the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C symmetric stretching from the thiophene ring. The C–S bending mode occurred at 689 cm−1 for P3TArH and 639 cm−1 for nanocomposites. Meanwhile, Fe–O stretching modes occurred at 530–632 cm−1.31 Hence, the FT-IR study clearly revealed the functionalization of Fe3O4 NPs with P3TArH.
C symmetric stretching from the thiophene ring. The C–S bending mode occurred at 689 cm−1 for P3TArH and 639 cm−1 for nanocomposites. Meanwhile, Fe–O stretching modes occurred at 530–632 cm−1.31 Hence, the FT-IR study clearly revealed the functionalization of Fe3O4 NPs with P3TArH.
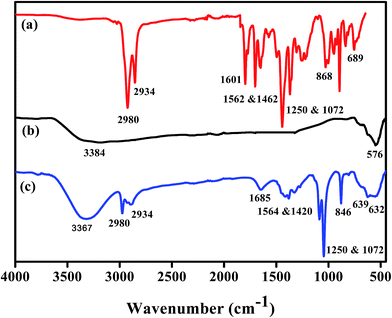 |
| | Fig. 1 FT-IR of (a) P3TArH, (b) Fe3O4, (c) Fe3O4@P3TArH. | |
3.1.2 XRD analysis. Fig. 2 shows the XRD pattern which revealed presence of the characteristic peaks of Fe3O4 in the synthesized materials. The peak of the nanocomposites was slightly broader than the ones corresponding to Fe3O4. This could be due to the presence of amorphous and polymeric materials.32 The distinctive peaks of Fe3O4 and nanocomposites were observed at 2θ = 30°, 35.7°, 43°, 53.4°, 57.0°, and 62.6°, which are marked by their respective indices [(220), (311), (400), (422), (511), and (440)].33 This indicated that the coating process with P3TArH does not change the crystalline phase of Fe3O4.
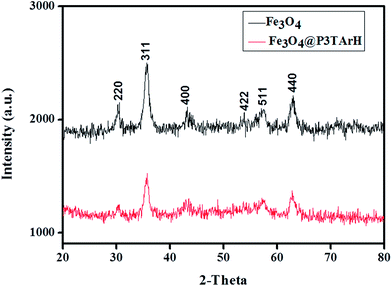 |
| | Fig. 2 XRD of Fe3O4 and Fe3O4@P3TArH. | |
3.1.3 Morphological analysis. Fig. 3 demonstrates morphological analysis of the synthesized products using FESEM and TEM techniques. FESEM and TEM images of all materials exhibited a sphere-shaped geometry. As evident from the TEM analysis, the distribution of the modified nanoparticles (Fe3O4@P3TArH) is very uniform and nanoparticles are segregated. This may be due to the presence of polymeric material coating, which reduces the aggregation and stabilizes the magnetic nanoparticles.21 Analysis of TEM images using the IMAGEJ software determined the average of particle diameter distribution by computing the values corresponding to at least 300 nanoparticles. Based on the histogram plotted in Fig. 3(e) and (f), DTEM, an average diameter, and σ (standard deviation values) were calculated. TEM average particle size for Fe3O4@P3TArH was found to be 13.070 nm ± 2.916. The average particle size Fe3O4 was larger than the average particle size of Fe3O4@P3TArH as determined from the TEM; this may be due to the fact that many nanoparticles are accumulated and overlaid on top of one another, and thus cannot be measured accordingly.32
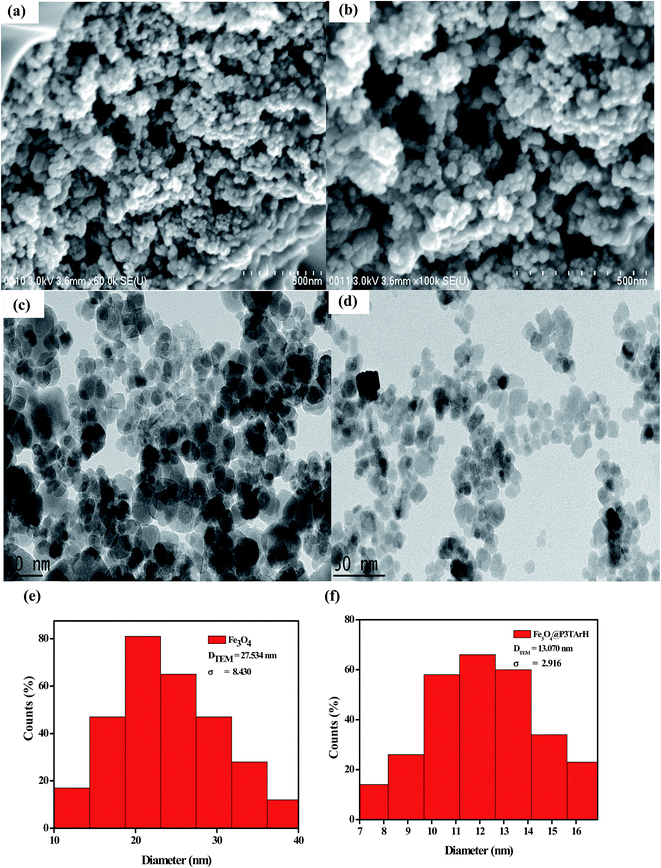 |
| | Fig. 3 FESEM images of (a) Fe3O4; (b) Fe3O4@P3TArH, TEM images of (c) Fe3O4; (d) Fe3O4@P3TArH and diameter distribution of (e) Fe3O4; (f) Fe3O4@P3TArH. | |
3.1.4 VSM analysis. Magnetic properties of the samples were recorded at room temperature with an external field of ±15 kOe. M–H hysteresis curves of Fe3O4 and Fe3O4@P3TArH are presented in Fig. 4. An important magnetic parameter, which is saturation magnetization (MS), was assessed. As is clear from the hysteresis loops, magnetization did not occur until the maximum applied field and exhibited superparamagnetic behavior.34 Maximum saturation (MS) of Fe3O4 and Fe3O4@P3TArH appeared at 92 (bulk magnetization) and 63.2 emu g−1, respectively. Reduced magnetization signifies the presence of a dead magnetic layer on the surface of the nanocomposites.31 Magnetization is reduced in nanocomposite, but still the value of magnetization falls within the acceptable range, which suggested that the nanocomposites still can be separated conveniently from a solution with an external magnetic field.35
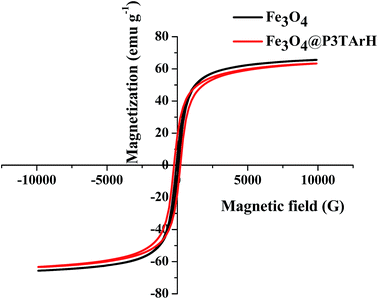 |
| | Fig. 4 VSM spectra of Fe3O4 and Fe3O4@P3TArH. | |
3.1.5 Thermal analysis. Fig. 5 shows TG and DTG analyses for Fe3O4 and Fe3O4@P3TArH. A small weight loss was detected under 200 °C in Fe3O4 and was speculated to be due to desorption of adsorbed water on the surface of the nanoparticles. Since Fe–O is thermodynamically steady within a temperature range of 280 °C to 850 °C, no weight loss was observed for Fe3O4 after 200 °C.36 The TGA thermogram of the nanocomposite indicated that it is stable up to 210 °C. However, above this temperature Fe3O4@P3TArH exhibited rapid weight loss in the temperature range from 240 °C and 450 °C. This might be due to the decomposition of P3TArH. Herein, thermal analysis study showed that the surface of Fe3O4 was successfully coated with P3TArH.
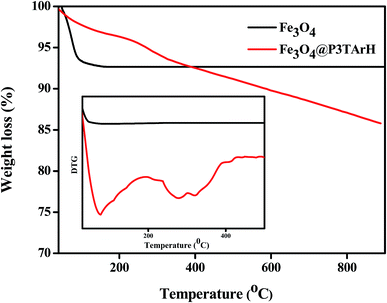 |
| | Fig. 5 TGA and DTG (inset) thermograms of Fe3O4, and Fe3O4@P3TArH. | |
3.1.6 N2 physisorption analysis. The BET surface area was measured using the multipoint BET method within the relative pressure (P/P0) range of 0.05 to 1. As described in Fig. S5 (ESI†), the Fe3O4 and Fe3O4@P3TArH displays a H3 type hysteresis loop, based on the Brunauer–Deming–Deming–Teller (BDDT) classification, indicating the presence of mesopores with pore diameters between 2 and 50 nm.37 The pore size and BET surface area of both Fe3O4 and nanocomposites are tabulated in Table 1. The decrease in pore size of nanocomposites is due to the addition of polymer coating on the surface. Increase in the surface area may be attributed to the aggregation of particles that resulted in the enhancement of the spaces between them.38,39
Table 1 BET pore size and surface area
| Sample |
Pore size (nm) |
Surface area (m2 g−1) |
| Fe3O4 |
20.2 |
37.37 |
| Fe3O4@P3TArH |
12.09 |
103.80 |
3.2 Adsorptive performance
3.2.1 Type of adsorbent. The adsorption efficiency of DEHP for three different types of sorbent namely, Fe3O4, polythiophene coated Fe3O4 (Fe3O4@PTh), and Fe3O4@P3TArH is shown in Fig. 6. As evident from the figure, Fe3O4 showed adsorption efficiency of 32.52%, while with Fe3O4@PTh the value increased to 46.93%. After coating the surface of Fe3O4 with P3TArH, the adsorption efficiency increased to 68.73%. This might be due to the presence of more active sites in the Fe3O4@P3TArH as well as various physical interactions between adsorbent and adsorbate (hydrophobicity and π–π interaction) as compared to other sorbents, which enhanced the adsorption of DEHP.40
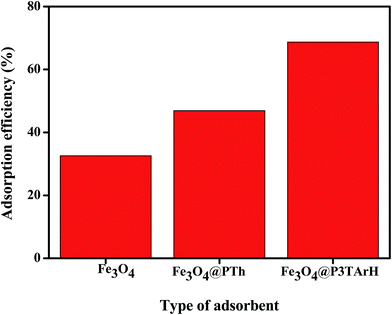 |
| | Fig. 6 Type of adsorbent (DEHP concentration, 15 mg L−1; adsorbent, 1 mg L−1; contact time, 60 min; pH 7). | |
3.2.2 Effect of pH. Adsorption was performed under different pH conditions, ranging from pH 3–10. As evidenced from Fig. 7(a), adsorption efficiency increased when the pH increased from 5 to 7, but decreased later from 7 to 10. Fig. 7(b) demonstrated zeta potential analysis of Fe3O4@P3TArH with effects of pH range 3 to 9 which represented the surface charge of Fe3O4@P3TArH. At low pH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups in Fe3O4@P3TArH were protonated, making the adsorbent surface positively charged. As described in Scheme 2, at pH < 7, DEHP hydrolyzes to phthalic acid; since the carbonyl group in the phthalic acid is nucleophilic in nature, it can easily react with hydrogen ions in the solution to form positively charged species. Due to both adsorbate and adsorbent acquiring positive charges, electrostatic repulsion occurred and retarded the adsorption performance.41 Moreover, based on zeta potential results at higher pH, the surface of the adsorbent became negatively charged due to deprotonation of C
N groups in Fe3O4@P3TArH were protonated, making the adsorbent surface positively charged. As described in Scheme 2, at pH < 7, DEHP hydrolyzes to phthalic acid; since the carbonyl group in the phthalic acid is nucleophilic in nature, it can easily react with hydrogen ions in the solution to form positively charged species. Due to both adsorbate and adsorbent acquiring positive charges, electrostatic repulsion occurred and retarded the adsorption performance.41 Moreover, based on zeta potential results at higher pH, the surface of the adsorbent became negatively charged due to deprotonation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in Fe3O4@P3TArH, whereas the adsorbate hydrolyzed to phthalate anions as shown in Scheme 3, resulting in repulsion and, thereby, reduced the adsorption efficiency.42 Strong adsorptive performance in neutral pH can be explained by strong interactions between the hydrophobic and π–π interactions in Fe3O4@P3TArH with DEHP. Since the optimum performance of adsorption of DEHP is demonstrated at pH 7, this pH was selected for all of the experiments.
N in Fe3O4@P3TArH, whereas the adsorbate hydrolyzed to phthalate anions as shown in Scheme 3, resulting in repulsion and, thereby, reduced the adsorption efficiency.42 Strong adsorptive performance in neutral pH can be explained by strong interactions between the hydrophobic and π–π interactions in Fe3O4@P3TArH with DEHP. Since the optimum performance of adsorption of DEHP is demonstrated at pH 7, this pH was selected for all of the experiments.
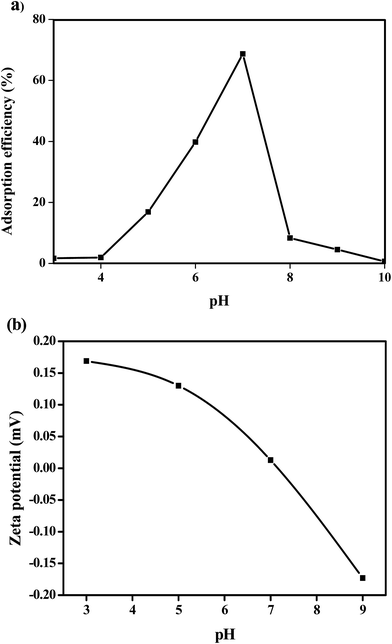 |
| | Fig. 7 (a) Effect of solution pH (DEHP concentration, 15 mg L−1; adsorbent, 1 mg L−1; contact time, 60 min); (b) the zeta potential of Fe3O4@P3TArH at various pHs. | |
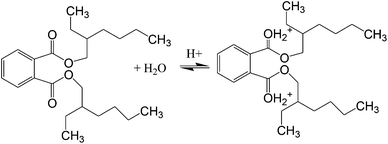 |
| | Scheme 2 DEHP at pH < 7. | |
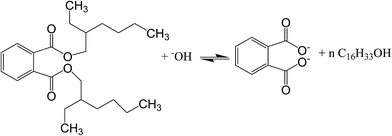 |
| | Scheme 3 DEHP at pH > 7. | |
3.2.3 Kinetic and thermodynamic studies. The effect of contact time for the removal of DEHP by Fe3O4@P3TArH was investigated in the time range of 0–180 min at four different temperatures of 298.15 K, 318.15 K, 323.15 K, and 333.15 K. Fig. 8 demonstrated that the adsorption capacity was rapid for the first 15 min; which might be due to the many available active sites for adsorption. From 120 min to 180 min the removal capacity was observed to be constant; therefore, 120 min could be regarded as the equilibrium time. To further determine the adsorption mechanism and kinetics parameters, the data gained were fitted into three types of kinetic models: pseudo first-order, pseudo second-order, and intraparticle diffusion.43–47 The pseudo first-order kinetic model was extensively used to study the adsorption of an adsorbate from an aqueous solution. The equation can be expressed as;| |
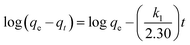 | (3) |
where qe and qt are the amount of DEHP adsorbed (mg g−1) at equilibrium and time, t is the contact time (min), and k1 is the rate constant of this equation (min−1). k1 and qe were determined from the plot of log(qe − qt) versus t. Meanwhile, a pseudo-second order kinetic model is based on the assumption that the adsorption mechanism depends on both adsorbate and adsorbent.48 The linear equations pertaining to this area follows;| |
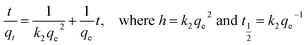 | (4) |
where h is the initial adsorption rate (mg g−1) min, t1/2 is half equilibrium time (min), and k2 is the pseudo second-order rate constant (g mg−1 min). A linear plot of t/qt vs. t could be used to determine the value of qe, k2, h, and t1/2 respectively. The kinetics parameters and correlation coefficients are listed in Table 2. It can be seen that the experimental data is fitted with the pseudo second-order kinetic model with R2 near to unity and can be further confirmed by the nearness of the calculated and experimental qe value. Similar adsorption behavior is also found for the removal of diethyl phthalate by activated carbon.49 Next, the parameters for intraparticle diffusion models were calculated according to the linear equation as follows;where c is thickness of boundary layer (mg g−1) and K is the rate constant (mg g−1 min−1). Fig. 9 reveals that the plots are not linear throughout the contact time. However, a similar trend for four temperatures were observed, and the plots were divided into two parts: at the start of the experiment, a steeper line is detected, which might be due to a massive transfer of DEHP from the boundary layer to the surface of the Fe3O4@P3TArH. After 8 min the line continued to be linear, due to diffusion of DEHP through the mesopores of Fe3O4@P3TArH.50
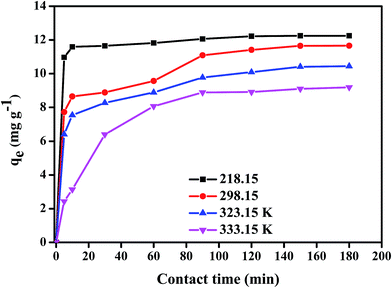 |
| | Fig. 8 Effect of contact time and temperature (DEHP concentration, 15 mg L−1; adsorbent, 1 mg L−1; pH 7). | |
Table 2 Kinetics parameters of DEHP adsorption onto Fe3O4@P3TArH
| Kinetic models |
Fe3O4@P3TArH |
| 298.15 K |
318.15 K |
323.15 K |
333.15 K |
| Pseudo-first order |
| qe,exp (mg g−1) |
12.22 |
11.413 |
10.09 |
8.914 |
| qe,cal (mg g−1) |
10.6 |
7.45 |
6.06 |
11.57 |
| k1 (min−1) |
0.0363 |
0.0317 |
0.0221 |
0.0603 |
| R2 |
0.7296 |
0.8754 |
0.8193 |
0.9216 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Pseudo-second order |
| qe,cal (mg g−1) |
12.30 |
11.89 |
10.60 |
9.85 |
| k2 (min−1) |
0.083 |
0.015 |
0.014 |
0.008 |
| R2 |
0.9999 |
0.9957 |
0.9936 |
0.9926 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Intraparticle diffusion |
| K (mg g−1 min−1) |
0.831 |
0.744 |
0.695 |
0.692 |
| c (mg g−1) |
3.278 |
3.251 |
3.214 |
1.321 |
| R2 |
0.705 |
0.842 |
0.957 |
0.9057 |
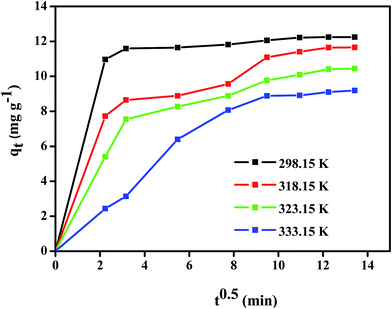 |
| | Fig. 9 Intraparticle diffusion plots. | |
From the intraparticle diffusion model parameters, the adsorption of DEHP onto Fe3O4@P3TArH may be assumed as an exothermic reaction, since the value of boundary layer thickness decreases with increasing temperature.51 Higher temperature resulted in the solubility of DEHP in aqueous solution increasing and that increased the collision between adsorbent molecules which led to a lower intraparticle diffusion rate.52
The thermodynamic parameters were determined to investigate the mechanism of the adsorption process. The values of adsorption enthalpy (ΔH°), entropy (ΔS), and Gibbs free energy (ΔG°), as shown in Table 3, were calculated using the following equations;
| |
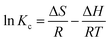 | (6) |
| |
 | (7) |
| |
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc Kc
| (8) |
Table 3 Thermodynamic parameters for DEHP adsorption onto Fe3O4@P3TArH
| T (K) |
qe,exp (mg g−1) |
ΔG° (kJ mol−1) |
ΔH° (kJ mol−1) |
ΔS (kJ mol−1 K−1) |
Ea (kJ mol−1 K−1) |
| 298.15 |
12.22 |
−2.883 |
−19.77 |
−0.057 |
−40.6 |
| 318.15 |
11.41 |
−1.650 |
| 323.15 |
10.09 |
−1.151 |
| 333.15 |
9.10 |
−0.881 |
|
|
|
From the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc against 1/T, the (ΔH°) value was found to be negative, which confirms that the adsorption process is exothermic in nature. The increase in temperature enhanced the surface activity of DEHP, increasing its affinity to water.53 The (ΔS) value was negative, demonstrating that the system reached an ordered state due to the decrease in the randomness of adsorbate/aqueous phase.54 Decreased ΔG° values with elevating temperature indicated that the process favors low temperature.55 Moreover, negative values of ΔG° were in a range between −20 and 0 kJ mol−1, indicating that the adsorption process is thermodynamically feasible, physically controlled, and spontaneous.56,57 The activation energy of the adsorption process can be calculated from the plot of k2 (pseudo second order rate constant) against 1/T using the Arrhenius equation;
Kc against 1/T, the (ΔH°) value was found to be negative, which confirms that the adsorption process is exothermic in nature. The increase in temperature enhanced the surface activity of DEHP, increasing its affinity to water.53 The (ΔS) value was negative, demonstrating that the system reached an ordered state due to the decrease in the randomness of adsorbate/aqueous phase.54 Decreased ΔG° values with elevating temperature indicated that the process favors low temperature.55 Moreover, negative values of ΔG° were in a range between −20 and 0 kJ mol−1, indicating that the adsorption process is thermodynamically feasible, physically controlled, and spontaneous.56,57 The activation energy of the adsorption process can be calculated from the plot of k2 (pseudo second order rate constant) against 1/T using the Arrhenius equation;
| |
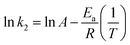 | (9) |
The value of activation energy for the adsorption of DEHP onto Fe3O4@P3TArH was found to be −40.6 kJ mol−1 K−1. The negative value of sorption activation energy demonstrated that the process favors low temperature.58 Moreover, the value of activation energy could be used to determine the adsorption behavior; if the values are less than <40 kJ mol−1, then the process is physisorption, and if it's >40 kJ mol−1, then it is predominantly a chemical adsorption process.
3.2.4 Effect of initial DEHP concentration and isotherm equilibrium studies. Fig. 10 demonstrates the effect of initial concentrations of DEHP in the range of 5–90 mg L−1 at 298 K. The adsorptive efficiency increased with increasing initial concentration of DEHP from 5 mg L−1 and equilibrium was reached at 15 mg L−1 with 83.4%. This indicated that a maximum concentration for 5 mg of Fe3O4@P3TArH in 5 mL aqueous solution is 15 mg L−1 DEHP, giving significant adsorption efficiency. At low concentration the removal is significant due to the readiness of many active sites for adsorption, whereas at high concentration the removal is persistent due to all the available active sites in adsorbent being fully occupied by DEHP.
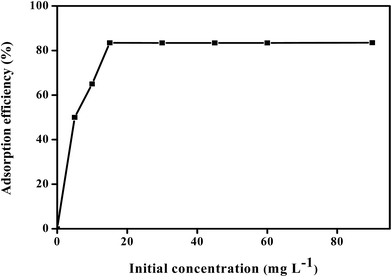 |
| | Fig. 10 Effect of DEHP initial concentration (contact time 60 min; adsorbent, 1 mg L−1; pH 7, 298.15 K). | |
To study the adsorption isotherm, four models (Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D–R)) were utilized to explain the adsorption behaviour of DEHP onto Fe3O4@P3TArH. The Langmuir isotherms model is based on the expectations that the monolayer adsorption homogenously occurred on all active sites, with no additional adsorption after the sites are fully occupied by the solute at uniform energy.59 The Langmuir equation is,
| |
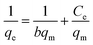 | (10) |
where
Ce (mg L
−1) is the equilibrium concentration of the adsorbate,
Co (mg L
−1) is the initial adsorbate concentration,
qe (mg g
−1) is the adsorption capacity at equilibrium,
qm (mg g
−1) and
b (L mg
−1) is the Langmuir constant related to the adsorption capacity and rate of adsorption, respectively. To determine whether the process is either favourable or unfavourable, the dimensionless separation factor (
RL) can be calculated from;
| |
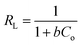 | (11) |
From the plot of Ce/qe versus Ce, the values of Langmuir parameters were determined and listed in Table 4. In contrast to the Langmuir isotherm model, the Freundlich isotherm model assumed that adsorption heterogeneously occurred on the adsorbent's surfaces, with different energies of active sites.60 The following equations determined the values of Freundlich constant;
| |
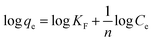 | (12) |
where
KF ((mg g
−1) (L mg
−1)
1/n) is the adsorption capacity, while 1/
n represents Freundlich constants, respectively. The Dubinin–Radushkevich isotherm model takes into account the porosity of the adsorbent. The experimental data was fitted with a plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe
qe against
ε2 as a linear equation in the following form;
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − βε2 qm − βε2
| (13) |
where
β (mol
2 kJ
−2) denotes the adsorption energy constant and the Polanyi potential (
ε) is mean free energy respectively.
E (kJ mol
−1) can be obtained using the following equations;
| |
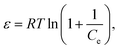 | (14) |
| | |
E (kJ mol−1) = (2β−0.5)
| (15) |
where
R is the universal gas constant in kJ mol
−1 K
−1, and
T is the temperature. The Temkin model was established on the basis that the heat of adsorption would reduce linearly as the layer is covered, resulting in an uneven relationship of adsorbent–adsorbate interfaces on heterogeneous surfaces in adsorption systems.
61 The Temkin model linear equation is as follows:
| |
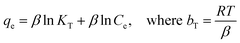 | (16) |
Table 4 Isotherms parameters for DEHP adsorption of onto Fe3O4@P3TArH
| Isotherms model |
Fe3O4@P3TArH |
| Langmuir |
| qm (mg g−1) |
52.63 |
| b (L mg−1) |
0.010 |
| R2 |
0.8259 |
| RL |
0.8695 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Freundlich |
| KF ((mg g−1) (L mg−1)1/n) |
0.6945 |
| 1/n |
1.168 |
| R2 |
0.9833 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Temkin |
| KT (L mg−1) |
0.217 |
| bT (kJ mol−1) |
276.88 |
| R2 |
0.9682 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Dubinin–Radushkevich |
| qm (mg g−1) |
20.00 |
| β |
10.575 |
| E |
0.615 |
| R2 |
0.8502 |
A plot of qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce from the linear equation could be used to determine the Temkin constant, where KT (L mg−1) is the Temkin constant related to the equilibrium binding energy, and bT (J mol−1) is Temkin constant related to the heat of adsorption.
Ce from the linear equation could be used to determine the Temkin constant, where KT (L mg−1) is the Temkin constant related to the equilibrium binding energy, and bT (J mol−1) is Temkin constant related to the heat of adsorption.
Amid the four isotherms studied, the result showed that the adsorption of DEHP onto Fe3O4@P3TArH was better described by Freundlich isotherm models (R2 = 0.9833), suggesting that adsorption occurred heterogeneously on adsorption sites. Comparable studies were also found for the adsorption of diethyl phthalate (DEP) using multiwall carbon nanotubes.62 Additionally, from the linear plot illustrated from Table 4 values, the Freundlich constant, adsorption capacity KF, and adsorption intensity 1/n value were determined to be 0.6945 mg g−1 and 1.168, respectively. A high KF value represented great adsorption affinity towards adsorbate,63 whereas 1/n ≥ 1 indicated favourable adsorption process.64 Fig. 11 demonstrated an illustration of proposed interaction for multilayer adsorption according to the Freundlich isotherms. When DEHP and Fe3O4@P3TArH come into contact, sorbent active sites which are mesopores (confirmed by BET), hydrophobicity (aliphatic) and π–π interaction (aromatic) enhance the attraction of DEHP. At the same time, DEHP–DEHP also attract each other causing multilayer adsorption which is often observed as a physical adsorption process.49,65
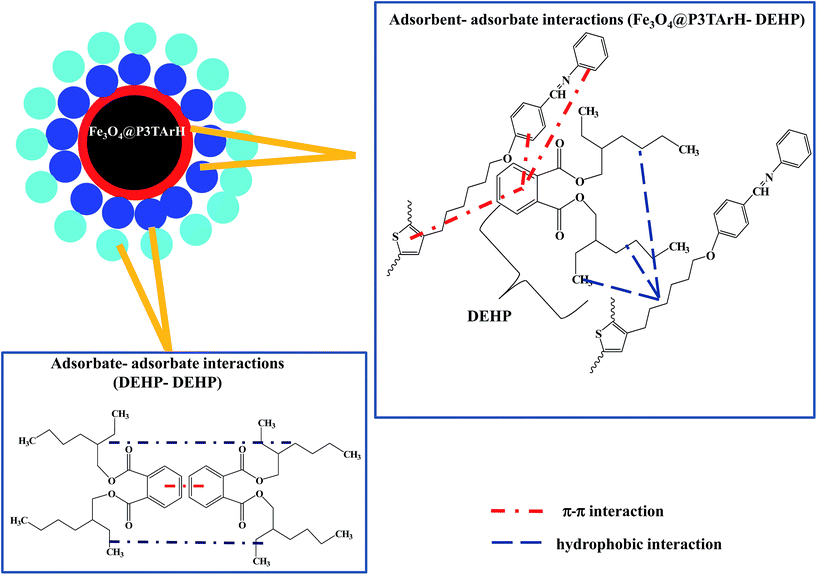 |
| | Fig. 11 Proposed interactions for multi-layer adsorption (Freundlich). | |
3.3 Reusability studies
To determine reusability of Fe3O4@P3TArH for adsorption of DEHP, the adsorbent was recycled after being washed with methanol and water, and was dried in vacuum at 70 °C for 12 hours. From Fig. 12, it could be summarized that after five repeated experiments, the adsorption performance was reduced from 84% to 64%. To further confirm the modifications on the sorbent properties, BET surface area analysis and FT-IR of the sorbent was performed after the fifth cycles. From the surface area analysis in Table 5, it was found that surface area of the recycled sorbent was significantly reduced. This may be due to the surface modification of the sorbent material which occurred until fifth cycle as confirmed by FT-IR analysis of the recycled sorbent.66 The IR spectrum of the recycled sorbent is presented in Fig. 13, and clearly reveals that the intensity of the polymer coating was reduced significantly after the fifth cycle. As a result, active sites (mesopores) and other physical interactions, which played a significant role in adsorption (hydrophobicity, π–π interaction) of DEHP, were decreased from the recycled Fe3O4@P3TArH which eventually hampered its adsorptive performance with every cycle.
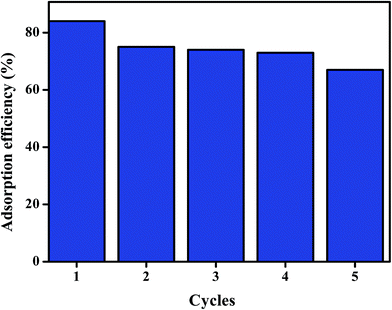 |
| | Fig. 12 Reusability graph for five cycles. | |
Table 5 BET surface area analysis of Fe3O4@P3TArH and recycled Fe3O4@P3TArH
| Sample |
BET surface area (m2 g−1) |
| Fe3O4@P3TArH |
103.8 |
| Recycled Fe3O4@P3TArH |
49.566 |
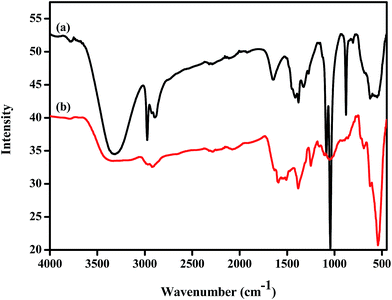 |
| | Fig. 13 FT-IR spectrum of (a) Fe3O4@P3TArH; (b) recycled Fe3O4@P3TArH. | |
3.4 Comparative studies
Table 6 reveals a comparison of the maximum adsorption capacities of various adsorbents. Chan H. et al. confirmed that breached seaweed resulted in higher adsorption capacity (5.68 mg g−1) compared to S. siliquastrum, (6.54 mg g−1) for an adsorbent mass of 0.025 g and 3 hours reaction time,67 Chen and Chung utilized chitosan bead as an adsorbent to remove DEHP from a plastic manufacturing plant. The maximum adsorption capacity gained from 1.5 g adsorbent dosage and a reaction time of 6 hours was 0.494 mg g−1.68 The adsorption capacity of DEHP (3.09 mg g−1) was enhanced by modifying chitosan via the introduction of α-cyclodextrin by the same author.69 Shailaja et al., who determined the maximum adsorption capacity was 0.97 mg g−1, removed DEHP through a soil slurry.70 A study conducted by Cao et al. using biofilms obtained 0.161 mg g−1 adsorption capacity with 1 g adsorbent.71 The value of qmax in this study was found to be 52.64 mg g−1, which suggests the Fe3O4@P3TArH used could readily adsorb DEHP.
Table 6 Comparison on adsorption capacities
| Adsorbent |
Analyte |
Adsorbent mass (g) |
Reaction time (hours) |
Maximum adsorption capacities (mg g−1) |
References |
| S. siliquastrum |
DEHP |
0.025 |
3 |
5.68 |
64 |
| Beached seaweed |
DEHP |
0.025 |
3 |
6.54 |
| Chitosan bead |
DEHP |
1.5 |
6 |
0.49 |
65 |
| Chitosan bead/α-cyclodextrin |
DEHP |
1.5 |
6 |
3.09 |
66 |
| Bioslurry |
DEHP |
15 |
264 |
0.972 |
67 |
| Biofilms |
DEHP |
1 |
— |
0.161 |
68 |
| Fe3O4@P3TArH |
DEHP |
0.01 |
2 |
52.63 |
This study |
4. Conclusion
Magnetic nanoparticles of Fe3O4 coated with modified polythiophenes (Fe3O4@P3TArH) were successfully synthesized and characterized. FTIR confirmed the presence of P3TArH on the surface of nanoparticles after surface modification. The adsorption processes were shown to be pH dependent, with the optimum removal being observed at a pH of 7. Kinetics analysis indicated that the kinetic data is well fitted in a pseudo second-order equation model. Thermodynamic studies revealed that the adsorption process was exothermic, spontaneous, and in an ordered state. The equilibrium isotherm data fit well into the Freundlich isotherm with 1/n indicating a favorable process. The adsorption suggests a multilayer adsorption behavior by considering π–π interaction and hydrophobic interaction of the Fe3O4@P3TArH with DEHP. Reusability studies suggested that after five cycles, surface modification of Fe3O4@P3TArH occurred; thus, retarding adsorption efficiency.
Acknowledgements
We acknowledge the financial support of the Institute of Research Management and Monitoring (PG044-2012B) University of Malaya and the Ministry of Higher Education for High Impact Research grant (HIR/MOHE/SC/F0031). We also acknowledge the financial support of the Universiti Teknologi MARA and Ministry of Higher Education Malaysia for funding PhD studies to one of the authors-cum-researchers, Siti Nor Atika Baharin.
References
- X. Li, X. Wang, L. Li, H. Duan and C. Luo, Talanta, 2015, 131, 354–360 CrossRef CAS PubMed.
- P. Serôdio and J. Nogueira, Water Res., 2006, 40, 2572–2582 CrossRef PubMed.
- H. Koch, L. Gonzalez-Reche and J. Angerer, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2003, 784, 169–182 CrossRef CAS.
- C. P. Feás, M. B. Alonso, E. Pena-Vazquez, P. H. Hermelo and P. Bermejo-Barrera, Talanta, 2008, 75, 1184–1189 CrossRef PubMed.
- A. G. Oomen, C. H. M. Versantvoort, M. R. Duits, E. van de Kamp and K. van Twillert, RIVM report 320102003/2004, Application of in vitro digestion models to assess release of lead and phthalate from toy matrices and azo dyes from textile, National Institute for Public Health and the Environment (RIVM), Netherland, 2004 Search PubMed.
- M. Castillo and D. Barceló, Anal. Chim. Acta, 2001, 426, 253–264 CrossRef CAS.
- J. A. Tickner, T. Schettler, T. Guidotti, M. McCally and M. Rossi, Am. J. Ind. Med., 2001, 39, 100–111 CrossRef CAS PubMed.
- M. Wagner and J. Oehlmann, Environ. Sci. Pollut. Res., 2009, 16, 278–286 CrossRef CAS PubMed.
- E. Tahmasebi, Y. Yamini, M. Moradi and A. Esrafili, Anal. Chim. Acta, 2013, 770, 68–74 CrossRef CAS PubMed.
- H. Ohtani, I. Miura and Y. Ichikawa, Environ. Health Perspect., 2000, 108, 1189–1193 CrossRef CAS PubMed.
- G. Latini, A. Verrotti and C. De Felice, Curr. Drug Targets: Immune, Endocr. Metab. Disord., 2004, 4, 37–40 CrossRef CAS.
- P. Foster, Int. J. Androl., 2006, 29, 140–147 CrossRef CAS PubMed.
- P. Kajitvichyanukul and N. Suntronvipart, J. Hazard. Mater., 2006, 138, 384–391 CrossRef CAS PubMed.
- W. Chao, C. Lin, I. Shiung and Y. Kuo, Chemosphere, 2006, 63, 1377–1383 CrossRef CAS PubMed.
- B. Chang, C. Yang, C. Cheng and S. Yuan, Chemosphere, 2004, 55, 533–538 CrossRef CAS PubMed.
- P. Thebault, J. Cases and F. Fiessinger, Water Res., 1981, 15, 183–189 CrossRef CAS.
- W. Zhang, Z. Xu, B. Pan, L. Lv, Q. Zhang, Q. Zhang, W. Du, B. Pan and Q. Zhang, J. Colloid Interface Sci., 2007, 311, 382–390 CrossRef CAS PubMed.
- M. Julinová and R. Slavík, J. Environ. Manage., 2012, 94, 13–24 CrossRef PubMed.
- S. Shahabuddin, N. Muhamad Sarih, S. Mohamad and s. n. a. baharin, RSC Adv., 2016 10.1039/c6ra04757b.
- D. Xiao, C. Zhang, D. Yuan, J. He, J. Wu, K. Zhang, R. Lin and H. He, RSC Adv., 2014, 4, 64843–64854 RSC.
- S. Shin and J. Jang, Chem. Commun., 2007, 4230–4232 RSC.
- J. Meng, J. Bu, C. Deng and X. Zhang, J. Chromatogr. A, 2011, 1218, 1585–1591 CrossRef CAS PubMed.
- A. Mehdinia, F. Roohi and A. Jabbari, J. Chromatogr. A, 2011, 1218, 4269–4274 CrossRef CAS PubMed.
- E. Tahmasebi and Y. Yamini, Microchim. Acta, 2014, 181, 543–551 CrossRef CAS.
- M. Behpour, S. Ghoreishi, N. Soltani and M. Salavati-Niasari, Corros. Sci., 2009, 51, 1073–1082 CrossRef CAS.
- S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M. Khan and G. Bouet, Corros. Sci., 2011, 53, 1484–1488 CrossRef CAS.
- A. Yurt, A. Balaban, S. U. Kandemir, G. Bereket and B. Erk, Mater. Chem. Phys., 2004, 85, 420–426 CrossRef CAS.
- W. Chen, Y. Chen, F. Li, L. Chen, K. Yuan, K. Yao and P. Wang, Sol. Energy Mater. Sol. Cells, 2012, 96, 266–275 CrossRef CAS.
- L. Shen, P. E. Laibinis and T. A. Hatton, Langmuir, 1999, 15, 447–453 CrossRef CAS.
- B. J. Vasanthi and L. Ravikumar, Open J. Polym. Chem., 2013, 3, 70–77 CrossRef CAS.
- M. Aydın, Z. Durmus, H. Kavas, B. Esat, H. Sözeri, A. Baykal, F. Yılmaz and M. S. Toprak, Polyhedron, 2011, 30, 1120–1126 CrossRef.
- L. F. Cótica, I. A. Santos, E. M. Girotto, E. V. Ferri and A. A. Coelho, J. Appl. Phys., 2010, 108, 064325 CrossRef.
- S. Giri, B. G. Trewyn, M. P. Stellmaker and V. S. Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 5038–5044 CrossRef CAS PubMed.
- J. Jayabharathi, P. Ramanathan, V. Thanikachalam and C. Karunakaran, New J. Chem., 2015, 39, 3801–3812 RSC.
- Z. Ma, Y. Guan and H. Liu, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3433–3439 CrossRef CAS.
- M. Mahdavi, M. B. Ahmad, M. J. Haron, F. Namvar, B. Nadi, M. Z. A. Rahman and J. Amin, Molecules, 2013, 18, 7533–7548 CrossRef CAS PubMed.
- K. S. W. Sing, Journal, 1985, 57, 603 CAS.
- Q. Wang, Y. F. Chen, M. Yang, X. F. Wu and Y. J. Tian, 2008.
- J. G. Darab, J. C. Linehan and D. W. Matson, Energy Fuels, 1994, 8, 1004–1005 CrossRef CAS.
- C. Moreno-Castilla, Carbon, 2004, 42, 83–94 CrossRef CAS.
- S. V. Mohan, N. C. Rao, K. K. Prasad and J. Karthikeyan, Waste Manag., 2002, 22, 575–582 CrossRef PubMed.
- Z. Fang and H. Huang, Adsorpt. Sci. Technol., 2009, 27, 685–700 CrossRef CAS.
- S. Memon, N. Memon, S. Memon and Y. Latif, J. Hazard. Mater., 2011, 186, 1696–1703 CrossRef CAS PubMed.
- B. Pan, B. Pan, W. Zhang, Q. Zhang, Q. Zhang and S. Zheng, J. Hazard. Mater., 2008, 157, 293–299 CrossRef CAS PubMed.
- J. Febrianto, A. N. Kosasih, J. Sunarso, Y.-H. Ju, N. Indraswati and S. Ismadji, J. Hazard. Mater., 2009, 162, 616–645 CrossRef CAS PubMed.
- Y.-S. Ho and G. McKay, Water Res., 2000, 34, 735–742 CrossRef CAS.
- W. J. Weber and R. R. Rumer, Water Resour. Res., 1965, 1, 361–373 CrossRef CAS.
- F. A. Pavan, S. L. Dias, E. C. Lima and E. V. Benvenutti, Dyes Pigm., 2008, 76, 64–69 CrossRef.
- S. Venkata Mohan, S. Shailaja, M. Rama Krishna and P. N. Sarma, J. Hazard. Mater., 2007, 146, 278–282 CrossRef CAS PubMed.
- A. A. Jalil, S. Triwahyono, S. H. Adam, N. D. Rahim, M. A. A. Aziz, N. H. H. Hairom, N. A. M. Razali, M. A. Abidin and M. K. A. Mohamadiah, J. Hazard. Mater., 2010, 181, 755–762 CrossRef CAS PubMed.
- P. Tewari, A. Campbell and W. Lee, Can. J. Chem., 1972, 50, 1642–1648 CrossRef CAS.
- K. Yadava, B. Tyagi and V. Singh, J. Environ. Sci. Health, Part A: Environ. Sci. Eng., 1989, 24, 783–808 CrossRef.
- G. Minling, M. Xiaojun, S. Wenhua, Q. Yun and W. Lin, Int. J. Environ. Res., 2015, 9, 605–612 CAS.
- Y. A. Aydın and N. D. Aksoy, Chem. Eng. J., 2009, 151, 188–194 CrossRef.
- N. Salleh, A. Jalil, S. Triwahyono, J. Efendi, R. Mukti and B. Hameed, Appl. Surf. Sci., 2015, 349, 485–495 CrossRef CAS.
- M. Kilic, E. Apaydin-Varol and A. E. Pütün, J. Hazard. Mater., 2011, 189, 397–403 CrossRef CAS PubMed.
- A. Zarrouk, B. Hammouti, H. Zarrok, S. Al-Deyab and M. Messali, Int. J. Electrochem. Sci., 2011, 6, 6261–6274 CAS.
- P. Saha and S. Chowdhury, Insight into adsorption thermodynamics, INTECH Open Access Publisher, 2011 Search PubMed.
- V. C. Srivastava, M. M. Swamy, I. D. Mall, B. Prasad and I. M. Mishra, Colloids Surf., A, 2006, 272, 89–104 CrossRef CAS.
- G. Mckay, H. Blair and J. Gardner, J. Appl. Polym. Sci., 1982, 27, 3043–3057 CrossRef CAS.
- M. I. Temkin and V. Pyzhev, Acta Physicochim. URSS, 1940, 12(3), 217–222 Search PubMed.
- F. Wang, J. Yao, K. Sun and B. Xing, Environ. Sci. Technol., 2010, 44, 6985–6991 CrossRef CAS PubMed.
- H. Xu, L. Yang, P. Wang, Y. Liu and M. Peng, J. Mater. Sci. Technol., 2007, 23, 417 CAS.
- L. Pelit, F. Ertaş, A. Eroğlu, T. Shahwan and H. Tural, Bioresour. Technol., 2011, 102, 8807–8813 CrossRef CAS PubMed.
- S. Lyubchik, A. Lyubchik, I. Fonseca, O. Lygina and S. Lyubchik, Comparison of the thermodynamic parameters estimation for the adsorption process of the metals from liquid phase on activated carbons, INTECH Open Access Publisher, 2011 Search PubMed.
- M. Cherian, M. S. Rao, W.-T. Yang, J.-M. Jehng, A. M. Hirt and G. Deo, Appl. Catal., A, 2002, 233, 21–33 CrossRef CAS.
- H. Chan, T. Lau, P. Ang, M. Wu and P. Wong, J. Appl. Phycol., 2004, 16, 263–274 CrossRef CAS.
- C.-Y. Chen and Y.-C. Chung, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2006, 41, 235–248 CrossRef CAS PubMed.
- C.-Y. Chen, C.-C. Chen and Y.-C. Chung, Bioresour. Technol., 2007, 98, 2578–2583 CrossRef CAS PubMed.
- S. Shailaja, S. Venkata Mohan, M. Rama Krishna and P. N. Sarma, Int. Biodeterior. Biodegrad., 2008, 62, 143–152 CrossRef CAS.
- X. Cao, X. Li and X. Meng, Environ. Eng. Sci., 2014, 31, 55–60 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04172h |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 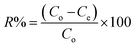
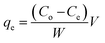
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) was observed at 1601 cm−1 for P3TArH, and 1685 cm−1 for the Fe3O4@P3TArH.30 C
N) was observed at 1601 cm−1 for P3TArH, and 1685 cm−1 for the Fe3O4@P3TArH.30 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic stretching peaks exhibited at 1562 cm−1 and 1462 cm−1 for P3TArH, whereas 1564 cm−1 and 1462 cm−1 for Fe3O4@P3TArH. Two absorption band peaks at 1250 and 1072 cm−1 indicated the presence of C–O observed in the P3TArH as well as Fe3O4@P3TArH. The strong absorption peaks at 868 cm−1 for P3TArH and 846 cm−1 for the nanocomposite indicated the presence of C
C aromatic stretching peaks exhibited at 1562 cm−1 and 1462 cm−1 for P3TArH, whereas 1564 cm−1 and 1462 cm−1 for Fe3O4@P3TArH. Two absorption band peaks at 1250 and 1072 cm−1 indicated the presence of C–O observed in the P3TArH as well as Fe3O4@P3TArH. The strong absorption peaks at 868 cm−1 for P3TArH and 846 cm−1 for the nanocomposite indicated the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C symmetric stretching from the thiophene ring. The C–S bending mode occurred at 689 cm−1 for P3TArH and 639 cm−1 for nanocomposites. Meanwhile, Fe–O stretching modes occurred at 530–632 cm−1.31 Hence, the FT-IR study clearly revealed the functionalization of Fe3O4 NPs with P3TArH.
C symmetric stretching from the thiophene ring. The C–S bending mode occurred at 689 cm−1 for P3TArH and 639 cm−1 for nanocomposites. Meanwhile, Fe–O stretching modes occurred at 530–632 cm−1.31 Hence, the FT-IR study clearly revealed the functionalization of Fe3O4 NPs with P3TArH.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups in Fe3O4@P3TArH were protonated, making the adsorbent surface positively charged. As described in Scheme 2, at pH < 7, DEHP hydrolyzes to phthalic acid; since the carbonyl group in the phthalic acid is nucleophilic in nature, it can easily react with hydrogen ions in the solution to form positively charged species. Due to both adsorbate and adsorbent acquiring positive charges, electrostatic repulsion occurred and retarded the adsorption performance.41 Moreover, based on zeta potential results at higher pH, the surface of the adsorbent became negatively charged due to deprotonation of C
N groups in Fe3O4@P3TArH were protonated, making the adsorbent surface positively charged. As described in Scheme 2, at pH < 7, DEHP hydrolyzes to phthalic acid; since the carbonyl group in the phthalic acid is nucleophilic in nature, it can easily react with hydrogen ions in the solution to form positively charged species. Due to both adsorbate and adsorbent acquiring positive charges, electrostatic repulsion occurred and retarded the adsorption performance.41 Moreover, based on zeta potential results at higher pH, the surface of the adsorbent became negatively charged due to deprotonation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in Fe3O4@P3TArH, whereas the adsorbate hydrolyzed to phthalate anions as shown in Scheme 3, resulting in repulsion and, thereby, reduced the adsorption efficiency.42 Strong adsorptive performance in neutral pH can be explained by strong interactions between the hydrophobic and π–π interactions in Fe3O4@P3TArH with DEHP. Since the optimum performance of adsorption of DEHP is demonstrated at pH 7, this pH was selected for all of the experiments.
N in Fe3O4@P3TArH, whereas the adsorbate hydrolyzed to phthalate anions as shown in Scheme 3, resulting in repulsion and, thereby, reduced the adsorption efficiency.42 Strong adsorptive performance in neutral pH can be explained by strong interactions between the hydrophobic and π–π interactions in Fe3O4@P3TArH with DEHP. Since the optimum performance of adsorption of DEHP is demonstrated at pH 7, this pH was selected for all of the experiments.

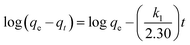
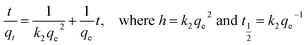

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
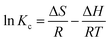

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc
Kc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc against 1/T, the (ΔH°) value was found to be negative, which confirms that the adsorption process is exothermic in nature. The increase in temperature enhanced the surface activity of DEHP, increasing its affinity to water.53 The (ΔS) value was negative, demonstrating that the system reached an ordered state due to the decrease in the randomness of adsorbate/aqueous phase.54 Decreased ΔG° values with elevating temperature indicated that the process favors low temperature.55 Moreover, negative values of ΔG° were in a range between −20 and 0 kJ mol−1, indicating that the adsorption process is thermodynamically feasible, physically controlled, and spontaneous.56,57 The activation energy of the adsorption process can be calculated from the plot of k2 (pseudo second order rate constant) against 1/T using the Arrhenius equation;
Kc against 1/T, the (ΔH°) value was found to be negative, which confirms that the adsorption process is exothermic in nature. The increase in temperature enhanced the surface activity of DEHP, increasing its affinity to water.53 The (ΔS) value was negative, demonstrating that the system reached an ordered state due to the decrease in the randomness of adsorbate/aqueous phase.54 Decreased ΔG° values with elevating temperature indicated that the process favors low temperature.55 Moreover, negative values of ΔG° were in a range between −20 and 0 kJ mol−1, indicating that the adsorption process is thermodynamically feasible, physically controlled, and spontaneous.56,57 The activation energy of the adsorption process can be calculated from the plot of k2 (pseudo second order rate constant) against 1/T using the Arrhenius equation;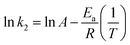

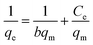
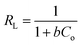
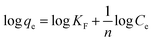
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe against ε2 as a linear equation in the following form;
qe against ε2 as a linear equation in the following form;![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln
qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − βε2
qm − βε2
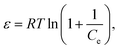
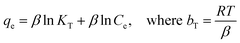
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce from the linear equation could be used to determine the Temkin constant, where KT (L mg−1) is the Temkin constant related to the equilibrium binding energy, and bT (J mol−1) is Temkin constant related to the heat of adsorption.
Ce from the linear equation could be used to determine the Temkin constant, where KT (L mg−1) is the Temkin constant related to the equilibrium binding energy, and bT (J mol−1) is Temkin constant related to the heat of adsorption.












