Control of pH by acetic acid and its effect on ethanol fermentation in an integrated ethanol–methane fermentation process†
Abstract
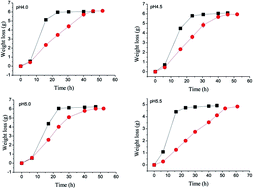
The integrated ethanol-methane fermentation process employing cassava or other feedstock requires pH control to maximize yields. Sulfuric acid is currently used to adjust the pH of the recycled process water but this causes problems with sulfate-reducing bacteria. To resolve this, acetic acid was evaluated to regulate pH in the range 4.0–5.5. Significant advantages were found and acetic acid toxicity was avoided. The ethanol yield from glucose increased 4.6% on average while production of the undesired byproduct glycerol decreased 52.6%. The ethanol yield per DCW (dry cell weight) increased from 14.0 g g−1 (sulfuric acid control) to 32.0 g g−1 when 250 mM acetic acid was present at pH 5.0. At pH 5.5, ethanol yield per DCW increased from 10.5 g g−1 to 23.0 g g−1. In both cases, the ethanol yield more than doubled. Using acetic acid instead of sulfuric acid to control pH thus increased ethanol production while simultaneously reducing undesirable side effects. The results presented here could also provide a useful approach for the management of wastewater in other submerged fermentation industries.

 Please wait while we load your content...
Please wait while we load your content...