Fabrication of a selective 4-amino phenol sensor based on H-ZSM-5 zeolites deposited silver electrodes
Abstract
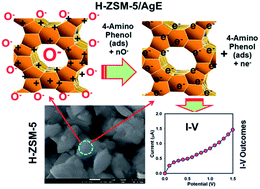
H-ZSM-5 zeolite is an inorganic material with large surface area and well-defined internal structure with porous uniform cages, cavities or channels. In this study, H-ZSM-5 was synthesised by calcination of the NH4-form at 500 °C for 3 h in air flow. This protonated H-ZSM-5 has been characterized in detail, which includes its optical, structural, morphological, and elemental properties by various conventional methods. For probable chemical sensor development, H-ZSM-5 was deposited on a silver electrode (AgE, surface area, 0.0216 cm2) to fabricate a sensor with a fast response towards selective 4-amino phenol (4-AMP) in the liquid phase. The sensor exhibited good sensitivity and long-term stability and enhanced electrochemical responses. The calibration plot was linear (r2 = 0.9979) over the 0.1 nM to 1.0 mM 4-AMP concentration ranges. The sensitivity was ∼2.085 μA cm−2 nM−1 and the detection limit was 0.02 nM (at a signal-to-noise ratio (SNR) of 3). By employing CV and EIS techniques, it was unveiled that the sensor is not well-operative in the absence of air. This shows a promising future for sensitive sensor development using mesoporous H-ZSM-5 by I–V methods for applications in the detection of hazardous and carcinogenic phenolic compounds in environmental and health care fields.

 Please wait while we load your content...
Please wait while we load your content...