In situ generated nickel on cerium oxide nanoparticle for efficient catalytic reduction of 4-nitrophenol†
Abstract
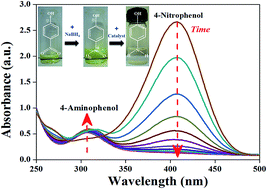
Efficient and economic catalysts are required for the large scale degradation of hazardous pollutants. In the present work, two nickel (5 wt%) based compounds, Ni(NO3)2 and NiO, immobilized over a CeO2 surface were tested for the reduction of 4-nitrophenol. Size, structural and surface properties of the catalyst were characterized by XRD, SEM & TEM – EDX, FTIR and Raman spectroscopy. UV-visible spectroscopic results indicated the better catalytic performance of the Ni(NO3)2 support than that of NiO supported CeO2. The reduction rate of 4-nitrophenol in the presence of the Ni(NO3)2 support was found to be 12 times faster than that of NiO supported CeO2. The time-dependent Raman spectroscopic investigation demonstrated that the performance of Ni(NO3)2 supported CeO2 arises from the in situ generation of nickel in the presence of an excess of sodium borohydride in the reduction of 4-nitrophenol. Further, the reversible conversion of nickel to nickel nitrate enabled the recyclability of the Ni(NO3)2 supported CeO2. The formation of nickel was found to be important for the reduction of 4-nitrophenol as NiO supported CeO2 did not form nickel thereby exhibiting poor catalytic activity. Thus, the present work showcases the in situ generation of nickel as a novel strategy for the catalytic reduction of 4-nitrophenol.

 Please wait while we load your content...
Please wait while we load your content...