Haiqin Liang, Zhiyi Yao, Wenqi Ge, Yadong Qiao, Li Zhang, Zhong Cao and Hai-Chen Wu
RSC Adv., 2016,6, 38328-38331
DOI:
10.1039/C6RA04080B,
Communication
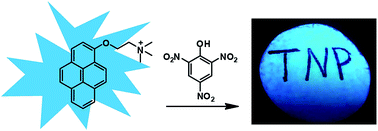
We have developed a fluorescent sensor based on a cationic pyrene derivative for the rapid detection of picric acid in aqueous media. It can be conveniently applied on test strips for visual detection of picric acid. The detection limit of this approach can be as low as 23.2 nM by fluorescence measurements.