Systemic research of fluorescent emulsion systems and their polymerization process with a fluorescent probe by an AIE mechanism†
Abstract
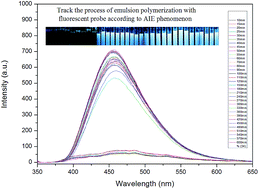
In this paper, an AIE luminogen, which was used as a fluorescent probe, was synthesized and copolymerized with acrylate monomers to study the process of emulsion polymerization and properties of a fluorescent emulsion. At first, according to the changes in the fluorescence spectra, the emulsion polymerization process can be followed with real-time monitoring. Then, by varying the relative content of the AIE luminogen, the glass transition temperature of the synthesized emulsion, the size of the emulsion particle, the contents of the emulsion, and the detection temperature, etc., the relationship between the fluorescence properties and intrinsic properties of the emulsion was studied systematically. It should be pointed out that the microscopic motion of a segment of polymer can be studied by fluorescence spectra with the help of a fluorescent probe. Traditionally, AIE luminogens are applied in optoelectronics and biological domains as small organic molecules. When an AIE luminogen is connected with polymer chains by a chemical bond, a lot of interesting phenomena can be observed. The research results not only provide a new method to study the emulsion polymerization process and properties of emulsion, but also, the synthesized emulsion with properties of fluorescence may broaden the application of the AIE mechanism.

 Please wait while we load your content...
Please wait while we load your content...