Specific detection of potassium ion in serum by a modified G-quadruplex method†
Shan Zhang‡
a,
Ruibin Zhang‡b,
Baojin Maa,
Jichuan Qiua,
Jianhua Lia,
Yuanhua Sanga,
Wei Liu*a and
Hong Liu*a
aState Key Laboratory of Crystal Materials, Shandong University, Jinan, 250100, China. E-mail: hongliu@sdu.edu.cn; weiliu@sdu.edu.cn
bBlood Purification Center, Jinan Central Hospital, Jinan, 250013, China
First published on 20th April 2016
Abstract
Potassium ion (K+) plays a central role in several fundamental physiological processes. Detection of the K+ concentration is an essential diagnostic tool for various medical diseases. However, most commercial detection methods are complex and expensive, which are not easily implemented in community hospitals or at home, in this study, we present a simple fluorescent K+ detection system based on the formation of G-quadruplex between K+ and dual-labelled thrombin aptamer oligonucleotide derivative (5′-FAM-TTTTTTAGGTTGGTGTGGTTGG-TAMRA-3′). Furthermore, based on this method, highly sensitive and selective detection of K+ in actual serum was realized by using EDTA as chelating agent to avoid the interference of Ca2+ and Mg2+ at physiological concentrations. Thus, this study paves the road toward the design and manufacture of portable potassium ions sensors based on fluorescence.
1. Introduction
The potassium ion, K+, plays a pivotal role in many essential functions of the human body, such as cellular transport, regulation of the membrane potential, maintenance of the blood pressure and muscle contraction.1–3 Several diseases related to the cardiovascular system, central nervous system and urinary tract are directly related to the K+ concentration outside its normal range.4,5 For uremia patients, the concentration of K+ must be measured at regular intervals in order to determine hemodialysis time and duration. Thus, concentration of K+ in serum is one of the major markers in medical diagnostics.Several commercial methods can be used to measure the concentration of K+, including flame luminosity, ion chromatography, use of ion-selective electrodes and dry chemistry methods.6–8 However, the existing potassium ion detection instruments are tedious, pricy and non-portable. Therefore, they are not suitable for community hospitals and home care.
To overcome these problems, the development of inexpensive, rapid, sensitive and selective detection methods has attracted considerable attention. Among those, methods based on the formation of G-quadruplex between K+ and a Thrombin Binding Aptamer (TBA) constitute a promising approach.9–12 In these methods, sensitive and effective detection of the G-quadruplex formation is essential for precise detection of K+ concentration. Although several strategies were explored to detect the formation of the G-quadruplex, including strategies of conjugating with a cationic polymer13,14 and strategies that monitor the fluorescence of a probe that either being anchored to the oligonucleotide15–17 or added externally,18,19 how to avoid the interference of other metal ions such as Ca2+, Mg2+ present in physiological serum is still a challenge.
In this work, as shown in Fig. 1, a dual-labelled TBA oligonucleotide derivative (5′-FAM-T TTT TTA GGT TGG TGT GGT TGG-TAMRA-3′) was selected to detect the K+ based on the occurrence of fluorescent resonance energy transfer (FRET)20 between FAM and TAMRA after the formation of G-quadruplex between TBA oligonucleotide and K+.21,22 Considering of that Ca2+ and Mg2+ could also induce the formation of G-quadruplex of TBA, a metal ion blocking agent was used to avoid their interference. Thus, K+ detection was achieved with high selectivity and high sensitivity. Furthermore, K+ in newborn calf serum (NBS), was successfully measured using this method, demonstrating the possibility of rapid K+ detection in serum based on formation of G-quadruples induced FRET.
2. Experimental section
2.1 Materials
TBA oligonucleotide derivative (5′-FAM-T TTT TTA GGT TGG TGT GGT TGG-TAMRA-3′) was synthesized by Shanghai Sangon Biological Engineering Technology & Service Co., Ltd. Other chemicals were all of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. Newborn calf serum (NBS) of New Zealand origin was bought from Gibco (product line of Thermo Fisher Scientific) and were stored frozen before use. The use of NBS was approved by the ethics committee of Shandong University.2.2 Measurements and methods
40 μl 5 μM dual-labelled thrombin binding aptamer (TBA) oligonucleotide was added into 3 ml KCl Tris–HCl solution at 5 mM. Then different amount of MgCl2 were added and got the solution containing 0.7, 0.8, 0.9, 1.0, 1.1 mM Mg2+ respectively and finally 10 mM EDTA-2Na were added. The fluorescence spectra of TBA and K+ mixture were measured at 25 °C under excitation of 490 nm.
3. Results and discussion
3.1 Relationship between fluorescence intensity and concentration of potassium ions
In this work, a dual-labelled TBA oligonucleotide derivative (5′-FAM-T TTT TTA GGT TGG TGT GGT TGG-TAMRA-3′) was designed for K+ detection. The ketonic oxygen in TBA oligonucleotide will combine with potassium ions and therefore the oligonucleotides fold into the form of G-quadruplex.23 When G-quadruplex forms between K+ and TBA oligonucleotide, two fluorophores FAM and TAMRA approach, and cause FRET occurrence. Fluorescence changes can be recorded by fluorescence spectrophotometer, which reflects the K+ concentration.15,16,21As shown in Fig. 2a, when no K+ in solution, there is fluorescence at 516 nm, but almost no fluorescence at 578 nm can be detected. However, when K+ exists, as the concentration of K+ in the dual labelled TBA increases, the fluorescent intensity at 516 nm representative of FAM decreases while the fluorescent intensity at 578 nm representative of TAMRA increases, indicating that the presence of K+ causes the formation of G-quadruplex between K+ and TBA, which induces occurrence of FRET from FAM to TAMRA. The variation of the fluorescence intensity at 516 nm and 578 nm reflects the occurrence of FRET between FAM and TARAM, which is induced by G-quadruplex formation after the addition of K+.24,25 Then the ration of fluorescence intensity at 516 nm and 578 nm was used to quantitatively evaluate the relationship between the between K+ concentration. As shown in Fig. 2b, the ration of fluorescence intensity, R (defined as R = I578 nm/I516 nm), increases linearly with the concentration of K+ in the 0–30 mM range, and the value of slope is 0.0040. As well known, normal K+ concentration in human serum is in the region of 3.5–5.5 mM,26 which is covered in the range of 0–30 mM designed in this method. These results indicates that dual-labelled TBA oligonucleotide based method is effective for K+ detection.
3.2 Interference of other metal ions on the detection of potassium ions
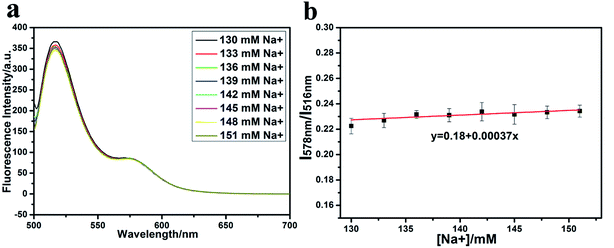
However, considering the existence of large amount of Na+, Mg2+ and Ca2+ in real serum, theses metal ions may have potential interference to K+ detection in real serum. So, the influence of Na+, Mg2+ and Ca2+ on the detection of K+ was further evaluated.As shown in Fig. 3a, with the addition of Na+, the fluorescence of dual-labelled TBA oligonucleotide at 516 nm decreases and that at 578 nm increases, which has a similar phenomenon to K+. What is different from K+ is that the variation of the fluorescence intensity is much lower with Na+ than with K+. As shown in Fig. 3b, the slope of the linear fit of R = I578 nm/I516 nm with Na+ concentration is only 0.00037, which is over 10 times smaller than that of K+ ions. Therefore, the interference of Na+ ions is negligible.
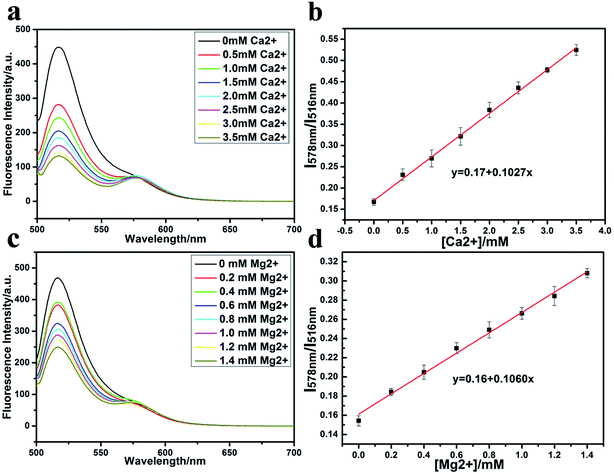
As for Mg2+ in the range of 0–1.4 mM (the Mg2+ concentration in human serum is 0.7–1.1 mM),31,32 the results are similar to those obtained with Ca2+ (Fig. 4c and d). The slope for Mg2+ is 0.1060, which is also about 24 times larger than for K+, thus indicating a strong interference of Mg2+ on the detection of K+ by the G-quadruplex fluorescence method.
3.3 Eliminating the interference of other metal ions for the detection of K+
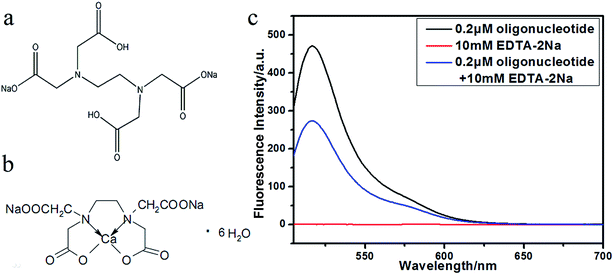
To eliminate the interference of Mg2+ and Ca2+, the most widely used chelating agent33,34 EDTA-2Na was used to sequester Ca2+ and Mg2+ (Fig. 5b).35–37 An EDTA-2Na concentration of 10 mM was selected in order to always be in excess compared to Ca2+ (2.25–2.75 mM) and Mg2+ (0.7–1.1 mM) in serum. Fig. 5c shows the fluorescence spectrum of the oligonucleotide, EDTA-2Na, and the oligonucleotide in the presence of EDTA-2Na. EDTA-2Na does not have fluorescence and the fluorescence of the oligonucleotide is only slightly weakened by the presence of EDTA-2Na.In order to check that whether EDTA-2Na can prevent the interference of Ca2+ and Mg2+ on the fluorescent K+ detection, the fluorescence of the dual-labelled oligonucleotide was measured in the presence of 10 mM EDTA-2Na, 5 mM K+ and various amounts of Ca2+ and Mg2+ (Fig. 6). When the Ca2+ concentration changes from 2.25 to 2.75 mM, there is no obviously changes of fluorescent intensity (Fig. 6a). The value of R is almost unchanged (Fig. 6b), and the slope of the linear equation is about 0, which indicates that, under these conditions, Ca2+ does not interfere with the fluorescence of the dual-labelled oligonucleotide. A similar situation occurs when the Mg2+ concentration increases from 0.7 to 1.1 mM (Fig. 6c and d).
The above results indicate that the addition of EDTA-2Na can successfully inhibit the combination of Ca2+ or Mg2+ with the oligonucleotide, without affecting the fluorescence of the G-quadruplex system.
3.4 Detection of potassium ions in simulated serum
To investigate the reliability of the K+ detection by the dual-labelled oligonucleotide, simulated serum solutions containing 145 mM Na+, 2.5 mM Ca2+, 0.9 mM Mg2+, 10 mM EDTA-2Na and K+ in concentrations ranging from 2.5 to 25 mM were prepared. The fluorescence spectra of the dual-labelled oligonucleotide G-quadruplex were measured, as shown in Fig. 7. The fluorescent intensities at 516 nm decreased and at 578 nm increased with increasing K+ concentration (Fig. 7a) and R is linearly correlated to the concentration of K+ (Fig. 7b). This test demonstrates the possibility of detecting K+ in the presence of Na+, Mg2+ and Ca2+.3.5 Detection of potassium ions in NBS
To demonstrate the possibility of dual-labeled TAB for K+ detection in blood, we detected the concentration of potassium ions in serum extracted from the blood of a newborn calf. The concentration of K+ ions in serum was measured by a cobas® 8000 automatic biochemistry analyzer beforehand, and the test result was 5.9 mM. The K+ concentration was tuned by adding standard K+ solution into the serum, thus getting samples with a gradient of K+ concentrations. To realize the measurements, 0.2 μM of dual-labelled oligonucleotides and 10 mM of EDTA-2Na were added. Furthermore, in order to test the efficiency of EDTA-2Na, the fluorescence of the solutions was also measured in the absence of EDTA-2Na. When no EDTA-2Na is present (Fig. 8a), the intensities of the fluorescent peaks at both 518 nm and 577 nm are unchanged when the K+ concentration varies, and the values of R = I577 nm/I518 nm are nearly identical (Fig. 8b). However, when EDTA-2Na is added to serum (Fig. 8c), the intensity of the fluorescent peak at 518 nm decreases with increasing K+ concentration while the peak at 577 nm increases which is consistent with the measurements above. Remarkably, the R value is linearly correlated to K+ concentration (Fig. 8d).The feasibility of developed sensor to detect K+ in serum using fluorescence spectrophotometer was investigated in spiked calf serum samples. Serum was diluted 3 times in 20 mM Tris–HCl buffer (pH = 7.4) containing 10 mM EDTA-2Na and then various concentrations of K+ were added. We can see from Table 1 that the recoveries were in the range of 96–101%. Therefore, the developed fluorescence sensor based on G-quadruplex is applicable for the determination of K+ in real samples.
| Background content (mmol l−1) | Added concentration (mmol l−1) | Detected concentration (mmol l−1) (n = 3) | Recovery ratio (%) |
|---|---|---|---|
| a Fluorescence response was measured in a calf serum sample diluted 3 times in 20 mM Tris–HCl buffer (pH = 7.4) containing 10 mM EDTA-2Na at 25 °C | |||
| 5.9 | 4 | 10.05 | 1.01 |
| 5.9 | 8 | 13.29 | 0.96 |
| 5.9 | 12 | 18.03 | 1.01 |
| 5.9 | 16 | 22.08 | 1.01 |
The comparison of properties with other existing method is shown in Table S1 in ESI.† T3TT3 sequences (5′-GGGTTTGGGTGGGTTTGGG) in method of crystal violet-G-quadruplex complexes have good selectivity to K+ when Na+ exists, but can not recognize K+ very well when Ca2+ and Mg2+ exist; Hum21 sequences (5′-GGGTTAGGGTTAGGGTTAGGG) in method of crystal violet-G-quadruplex complexes have good selectivity to K+ when Ca2+ and Mg2+ exist, but can not recognize K+ very well when Na+ exists. The linearity between fluorescence and concentration of K+ is good when high concentration of Na+ exists and this method can sense K+ in a range of 0–10 mM. The method of G-quadruplex with cationic conjugated polymer can recognize K+ well in the presence of Na+, but the interference of Ca2+ and Mg2+ can not be ignored. The linearity between fluorescence and concentration of K+ is good when Na+ exists and the limit of detection is 0–50 mM. Method of dual-labeled oligonucleotide derivative has studied the effects of spacer bases to the efficiency of FRET, and found that with the spacer bases TTTTTTA, the efficiency of FRET can be greatly improved. However, no further study works on the selectivity, linearity, and applicability of this dual-labeled oligonucleotide derivative system. Our method can detect K+ with Na+, Ca2+ and Mg2+ all existing and has perfect linearity even in real calf serum. The detection range is 0–30 mM which is large enough for K+ detection in serum. Above all, method of modified dual-labeled G-quadruplex in our study is most promising to be used in medical diagnosis.
To further explore the availability of this detection system in different conditions, we also tested the stability in different pH and temperature. As shown in Fig. S1,† the EDTA-2Na modified dual-labeled oligonucleotide system is nearly not affected by the variation of pH, so it is available at different pH. For the effect of temperature, the results indicate that R = I578 nm/I516 nm decreases with increase of measurement temperature, which indicates that the efficiency of FRET is lowered by the increasing temperature. It may be because of the equilibrium state of G-quadruplex is influenced by the increasing temperature and reduced the energy transfer. However, it would not influence the application of the detection system as we can optimize experimental parameters under different temperatures. Moreover, the temperature in human body is 36.5 to 37.5 °C, and this range would not affect very much during the detection.
As discussed above, the K+ detection method we developed using an EDTA-2Na standard solution to eliminate the interference of divalent ions only necessitates a fluorescence spectrophotometer, and can be adjusted to be available at different pH and temperatures. Therefore, this method is adapted to small-size portable devices for uses in hospitals or for family care.
4. Conclusion
Fluorescent-labeled TBA oligonucleotides offer a promising platform for the detection of K+. However, the selectivity of such system is quite low, as Ca2+ and Mg2+ at physiological concentrations also induce a change of conformation of the aptamer, resulting in an even larger FRET. The interference of Ca2+ and Mg2+ can simply be eliminated by adding EDTA-2Na which acts as a scavenger for these divalent ions. Thus, this modified dual-labelled G-quadruplex method has been successfully used to detect K+ in serum in the presence of Ca2+, Mg2+ and Na+ at their physiological concentrations. Therefore, this work connects the theory and practice of potassium ions fluorescent detection, and lays the foundations for the design and manufacture of a portable K+ sensor which would have a wide application in community hospitals and family care.Author contributions statement
The experiments were designed by Hong Liu and Wei Liu, and carried out by Shan Zhang and Ruibin Zhang, the results were analysed by Shan Zhang, Ruibin Zhang, Baojin Ma and Jichuan Qiu, Jianhua Li and Yuanhua Sang compiled the ESI.† Shan Zhang and Ruibin Zhang wrote the main manuscript text, Baojin Ma, Jichuan Qiu, Hong Liu and Wei Liu made significant contribution to the revision.Competing financial interests
The authors declare no competing financial interests.Acknowledgements
The authors are thankful for fundings from the National Natural Science Foundation of China (Grant No. 51372142). The Fundamental Research Funds of Shandong University (2014QY003, 2014JC019). Innovation Research Group (IRG: 51321091).References
- M. Wareing, et al., Expression and function of potassium channels in the human placental vasculature, Am. J. Physiol.: Regul., Integr. Comp. Physiol., 2006, 291, R437–R446 CrossRef CAS PubMed.
- R. C. Bahler, et al., Cardiovascular function in potassium-depleted dogs, J. Mol. Cell. Cardiol., 1971, 650–657 CAS.
- X. Nie, et al., Expression and insights on function of potassium channel TWIK-1 in mouse kidney, Pflügers Archiv - European Journal of Physiology, 2005, 451, 479–488 CrossRef CAS PubMed.
- H. Asita de Silva, et al., Abnormal function of potassium channels in platelets of patients with Alzheimer's disease, Lancet, 1998, 1590–1593 CrossRef.
- C. E. Harrison Jr, et al., Myocardial and mitochondrial function in potassium depletion cardiomyopathy, J. Mol. Cell. Cardiol., 1972, 633–649 CrossRef CAS.
- F. S. Chuang, et al., Flame spectrometric determination of sodium, potassium and calcium in blood serum by measurement of microsamples, Mikrochim. Acta, 1973, 523–531 CrossRef CAS.
- W. W. Buchberger, Detection techniques in ion chromatography of inorganic ions, TrAC, Trends Anal. Chem., 2001, 20, 6–7 CrossRef.
- A. S. Lima, et al., An Electrochemical Sensor Based on Nanostructured Hollandite-type Manganese Oxide for Detection of Potassium Ions, Sensors, 2009, 9(9), 6613–6625 CrossRef CAS PubMed.
- G. Biffi, et al., Visualization and selective chemical targeting of RNA G-quadruplex structures in the cytoplasm of human cells, Nat. Chem., 2014, 6, 75–80 CrossRef CAS PubMed.
- G. Biffi, et al., Quantitative visualization of DNA G-quadruplex structures in human cells, Nat. Chem., 2013, 5, 182–186 CrossRef CAS PubMed.
- S. M. Haider, et al., Structure of a G-quadruplex–Ligand Complex, J. Mol. Biol., 2003, 326, 117–125 CrossRef CAS PubMed.
- M. L. Bochman, K. Paeschke and A. Virginia, Zakian1, DNA secondary structures: stability and function of G-quadruplex structures, Nat. Rev. Genet., 2012, 13, 770–780 CrossRef CAS PubMed.
- F. He, et al., Fluorescent amplifying recognition for DNA G-quadruplex folding with a cationic conjugated polymer: a platform for homogeneous potassium detection, J. Am. Chem. Soc., 2005, 127, 12343–12346 CrossRef CAS PubMed.
- B. Kim, et al., Cationic conjugated polyelectrolytes-triggered conformational change of molecular beacon aptamer for highly sensitive and selective potassium ion detection, J. Am. Chem. Soc., 2012, 134, 3133–3138 CrossRef CAS PubMed.
- S. Nagatoishi, et al., Fluorescence energy transfer probes based on the guanine quadruplex formation for the fluorometric detection of potassium ion, Anal. Chim. Acta, 2007, 581, 125–131 CrossRef CAS PubMed.
- S. Nagatoishi, et al., Complexation of thrombin-binding aptamer oligonucleotide carrying fluorescence resonance energy transfer (FRET) dyes at both termini with potassium ion, Nucleic Acids Symp. Ser., 2005, 49, 233–234 CrossRef PubMed.
- C. Shia, et al., An aptamer-based fluorescent biosensor for potassium ion detection using a pyrene-labeled molecular beacon, Anal. Biochem., 2010, 400, 99–102 CrossRef PubMed.
- De-M. Kong, et al., Crystal violet-G-quadruplex complexes as fluorescent sensors for homogeneous detection of potassium ion, Biosens. Bioelectron., 2009, 25, 88–93 CrossRef PubMed.
- T. Li, et al., Parallel G-quadruplex-specific fluorescent probe for monitoring DNA structural changes and label-free detection of potassium ion, Anal. Chem., 2010, 82, 7576–7580 CrossRef CAS PubMed.
- Paul R. Selvin, The renaissance of fluorescence resonance energy transfer, Nat. Struct. Biol., 2000, 7(9), 730–734 CrossRef CAS PubMed.
- S. A. E. Marras, et al., Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes, Nucleic Acids Res., 2002, 30, 21 e122 CrossRef PubMed.
- C.-H. Lu, et al., A graphene platform for sensing biomolecules, Angew. Chem., 2009(121), 4879–4881 Search PubMed.
- J.-L. Mergny, Fluorescence energy transfer as a probe for tetraplex formation: the i-motif., Biochem., 1999, 38, 1573–1581 CrossRef CAS PubMed.
- Mu-Y. Kim, et al., Telomestatin, a Potent Telomerase Inhibitor That Interacts Quite Specifically with the Human Telomeric Intramolecular G-Quadruplex, J. Am. Chem. Soc., 2002, 124(35), 10278–10279 CrossRef PubMed.
- Y. Okamura, et al., Double-labeled donor probe can enhance the signal of fluorescence resonance energy transfer (FRET) in detection of nucleic acid hybridization, Nucleic Acids Res., 2000, 28(24), e107 CrossRef CAS PubMed.
- S. Sixou, et al., Intracellular oligonucleotide hybridization detected by fluorescence resonance energy transfer (FRET), Nucl. Acids Res., 1994, 22(4), 662–668 CrossRef CAS PubMed.
- J. E. Nordrehaug, et al., Serum potassium concentration as a risk factor of ventricular arrhythmias early in acute myocardial infarction, Circulation, 1985, 71, 645–649 CrossRef CAS PubMed.
- M. Vairamani and M. L. Gross, G-quadruplex formation of thrombin-binding aptamer detected by electrospray ionization mass spectrometry, J. Am. Chem. Soc., 2003, 125, 42–43 CrossRef CAS PubMed.
- S. Nagatoishi, et al., A Pyrene-Labeled G-Quadruplex Oligonucleotide as a Fluorescent Probe for Potassium Ion Detection in Biological Applications, Angew. Chem., 2005, 117, 5195–5198 CrossRef.
- T. M. Lerga and C. K. O'Sullivana, Rapid determination of total hardness in water using fluorescent molecular aptamer beacon, Anal. Chim. Acta, 2008, 610, 105–111 CrossRef CAS PubMed.
- Yi-Y. Yan, et al., Selective recognition of oncogene promoter G-quadruplexes by Mg2+, Biochem. Biophys. Res. Commun., 2010, 402, 614–618 CrossRef CAS PubMed.
- Yu-Y. Sun, et al., Increase in serum Ca2+/Mg2+ ratio promotes proliferation of prostate cancer cells by activating TRPM7 channels, J. Biol. Chem., 2013, 255–263 CrossRef CAS PubMed.
- E. A. Sausville, et al., Effect of chelating agents and metal ions on the degradation of DNA by bleomycin, Biochem, 1978, 17(14), 2740–2746 CrossRef CAS.
- S. Tandy, et al., Extraction of heavy metals from soils using biodegradable chelating agents, Environ. Sci. Technol., 2004, 38(3), 937–944 CrossRef CAS PubMed.
- C. Patton, et al., Some precautions in using chelators to buffer metals in biological solutions, Cell Calcium, 2004, 35, 427–431 CrossRef CAS PubMed.
- R. S. Waters, et al., EDTA chelation effects on urinary losses of cadmium, calcium, chromium, cobalt, copper, lead, magnesium, and zinc, Biol. Trace Elem. Res., 2001, 83(3), 207–221 CrossRef CAS PubMed.
- E. Keowmaneechai and D. J. McClements, J. Agric. Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCl2, Food Chem., 2002, 50(24), 7145–7153 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04046b |
| ‡ These authors contributed equally to this work and they should be regarded as co-first author. |
| This journal is © The Royal Society of Chemistry 2016 |