Corrosion behavior of 316L and 304 stainless steels exposed to industrial-marine-urban environment: field study†
P. Dhaiveegana,
N. Elangovanb,
T. Nishimurac and
N. Rajendran*a
aDepartment of Chemistry, Anna University, Chennai-600025, India. E-mail: nrajendran@annauniv.edu; Tel: +91-44-22358659
bDepartment of Chemistry, A. M. Jain College, Chennai-600114, India
cMaterial Recycling Design Group, Research Center for Strategic Materials, National Institute for Materials Science, Tsukuba, 305-047, Japan
First published on 27th April 2016
Abstract
The present investigation extensively compares the pitting corrosion behavior and mechanical stability of 316L and 304 stainless steels (SS) exposed to an Industrial-Marine-Urban (IMU) environment for 3 years from April 2012–March 2015. The surface morphology of the stainless steels exposed to the above environment was studied by atomic force microscopy (AFM) and scanning electron microscopy equipped with energy dispersive X-ray analysis (SEM-EDAX). Mechanical stability of the exposed samples was monitored by the Vickers microhardness test. Corrosion behavior of exposed samples was monitored by electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization studies. FT-Raman spectroscopy was employed for investigation of the atmospheric corrosion products; this offered much interesting information that eventually helped to understand the various corrosion phenomena that had occurred during the periods of 3, 6, 9, 12, 24, and 36 months to identify corrosion products which could not be evaluated from XRD analysis. The experimental results clearly show that both 316L and 304 SS were affected by pitting corrosion from the dissolution of Mn present in the alloy due to atmosphere electrolytes. Pit initiation occurs with the high amount of Cl− present as a highly corrosive agent. Hence, high pit density was observed in 304 because of the selective dissolution as MnS present in the alloying elements. However, presence of SO2 in the atmospheric location enhances pitting corrosion resistance by inducing a stable rust layer (Goethite) to form. Meteorological data and weight loss results clearly demonstrated that decreases in corrosion rate (which was due to the formation of a highly adherent passive film) occur remarkably during the dry season. The present investigation has observed a very high relative humidity (RH) value in the Chennai region of India. Very high RH reveals the presence of a thick water layer on the metal surface which acts as a sink to form the passive film. The electrochemical studies of atmospherically corroded 316L SS depicts passivation behavior whereas the 304 SS does not show any passivation behavior in the IMU region of Chennai.
1. Introduction
Austenitic stainless steel, because of its exceptional corrosion resistance, is widely used as an important and specific construction material in many fields and industries including electrical, construction, nuclear power, petrochemical, oil and gas, food processing, pharmaceutical, and transportation.1,2 For instance, 140 tons of 304 stainless steel was used as the construction material to build the historic building, Guildhall in London, which is estimated to have a life span of 750 years.3 Although SS is highly corrosion resistant, severe localized corrosion can occur in aggressive environments, especially in a marine atmosphere.4,5 When temperature drops and humidity rises in this environment, because of the moisture present in atmosphere, condensation forms tiny droplets or thin electrolyte layers containing chloride ions.6 But, in contrast when the temperature increases and relative humidity decreases, evaporation of water takes place with increase in chloride concentration at the surface layer.7 These chloride containing salts get deposited and play an important role in triggering localized corrosion in SS.6 When the environment is not exactly marine, e.g., if it is a mixed environment where industrial and urban populations co exist, then other atmospheric pollutants like SO2 play a significant role in atmospheric corrosion processes.8 There have been many previous studies carried out on long term and short term atmospheric corrosion processes using ATR, FT-IR, SEM, FT-Raman, XRD, and electroanalytical techniques.9,10 In the above techniques, Raman spectroscopy is a low cost tool to identify the corrosion products formed on field exposed SS samples.11 When the amount of the corrosion products formed is small, XRD cannot be used to identify them, but Raman spectroscopy is efficient enough to identify those formed in smaller amounts. Since multiple corrosion products are formed during the corrosion processes, Raman spectroscopy can easily identify the α, β, γ-FeOOH, and iron oxides with their characteristic signatures.12Apart from Raman spectroscopy, electrochemical impedance spectroscopy (EIS) has been increasingly used in the field of corrosion as a powerful tool to study surface conditions of metals. EIS data could fetch detailed information about the corrosion process and about changes in the resistive–capacitive nature of metal electrolyte, electrolyte/electrode interface. It is also a more effective tool for studying the localized corrosion via small pores.13
Previously, many researchers have carried out research to investigate atmospheric corrosion behavior of SS, especially in a marine environment and a few in mixed environments. Leban et al.14 reported the effect of surface roughness on corrosion properties of 304 SS in simulated marine and urban environments and concluded that corrosion rate increases with an increase in surface roughness. Lv et al.15 studied the corrosion behaviour of 304 SS in simulated marine environment and reported the pitting corrosion that took place was associated with dissolution of MnS and increased with an increase in exposure time. The effect of surface treatment viz., pickling, abrading, and annealing on 304 SS and its atmospheric corrosion resistance was compared with as received samples by Wallinder et al.16 in marine and urban environments. As received samples in urban environments exhibited high corrosion resistance and the abraded samples showed the lowest. Bright annealed samples exhibited higher corrosion resistance in a marine environment whereas abraded showed the lowest.
Erosion, in the manner of pitting corrosion, was reported by Dong et al.17 after exposure of 304 SS in Xisha marine atmosphere; increase in number and depth of pits along with increase in surface coverage with corrosion products was observed. The morphology of atmospheric pitting corrosion in 304L stainless steel plate was analyzed using MgCl2 droplets in relation to changes in relative humidity (RH) and chloride deposition density (CDD) by Steven et al.18 They found that the morphology is a sensitive function of RH and CDD.
Over the last two decades, atmospheric corrosion studies have been conducted by various researchers. In developing countries, like India, with wide geographical zones, (especially in regions like Chennai) corrosion studies have been involved with ranges of environment and meteorological conditions covering industrial-marine, rural, and marine-rural areas. A very few studies have been carried out and focused on the industrial-marine-urban environment in cities like Chennai, which is one among the main economical and industrial zones in India19 (Natesan et al. 2006). Various metals like aluminium, zinc, copper, Cu–Zn alloys (Cu–27Zn, Cu–20Zn, Cu–37Zn), mild steel (MS), galvanised iron (GI),20 low alloy steel, and plain carbon steels21–23 were exposed to severe environments to assess degradation and corrosion resistance behaviour.
In the absence of literature reports on atmospheric corrosion studies on 316L and 304 SS in Chennai, carrying out research in this arena will help society at large. Hence, in the present study we investigated atmospheric corrosion behavior of 316L and 304 SS field exposure for three years at Ennore, which is a mixed environment comprised of marine, industrial, and urban settings, using Raman and EIS techniques.
2. Materials
The 316L and 304 stainless steels were used as substrate materials for atmospheric corrosion exposure in an IMU environment. The chemical composition of the above steels is listed in Table 1, which was confirmed by inductively coupled plasma optical emission spectroscopy (ICP-OES).| Substrates/elements | C | Si | Mn | P | Cr | Ni | N | Mo | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| 316L | 0.03 | — | 1.95 | 0.02 | 17.2 | 12.6 | 0.05 | 2.40 | — | Balance |
| 304 | 0.06 | 0.46 | 1.32 | 0.03 | 18.2 | 09.1 | 0.04 | — | 0.04 | Balance |
2.1 Preparation and exposure of specimens
ASTM G 50-97 (1997)24 standard was followed for preparation of the specimens which were exposed to an IMU environment for 3 years. The dimensions of the specimens were 100 × 50 × 3 mm and abraded with 800# SiC paper, rinsed with water and acetone, dried, weighed, and kept in a moisture free desiccator for three days prior to exposure. The metal racks were made by 304 stainless steel with epoxy painting for better life span in an aggressive environment. Test specimens were mounted on the metal racks using nylon bolts and nuts to prevent a galvanic couple between each other at 30° to the horizontal facing towards the south side as shown in Fig. S1.† Exposed specimens were removed from the exposure site as per scheduled intervals of 3, 6, 9, 12, 24, and 36 months. Weight measurements were carried out before and after the appropriate exposure period. Specimens were kept 100 m from the marine and 200 m from the industrial environment. The topography map of the exposing the site is given in Fig. S2.†2.2 Measurement of atmospheric corrosion parameters
The climatic and environmental parameters viz., relative humidity, temperature, rainfall, and wind speed were obtained from the Regional Meteorological Centre (RMC), Government of India, Chennai. The corrosive agents viz., chloride and SO2 (available in the atmospheric site as determined using wet candle and sulphation plate analysis) were also reported on a yearly average basis (preparation of wet candle and sulphation plate and experimental procedure of the surface characterization is given in the supplementary information†).2.3 Electrochemical measurements
The electrochemical corrosion behaviour of bare and exposed specimens was subjected to polarization experiments in 3.5% NaCl solution. A conventional three electrode cell with steel as working electrode, platinum foil as counter electrode, and saturated calomel electrode (SCE) as a reference electrode was used. All the electrochemical tests were carried out at room temperature. All the steel specimens having an exposed surface area of 1 cm2 were used as the working electrode.3. Results and discussion
3.1 Corrosive agents and environmental characteristics in the exposure site (2012–2015)
The climatic parameters were obtained from RMC, Government of India, Chennai, and the results are summarized in Fig. S4.† The temperature values observed during the exposure period of study (April 2012 to March 2015) show that the average mean temperatures are generally higher during April to July. The high temperature was observed in the month of May (41 °C) (Fig. S4a†). During winter season, temperature remained low from December to February. There is some fluctuation at the exposure location since it is situated close to the coast. The average annual temperature was around 35 °C and the average relative humidity varied from 65 to 95 (%). Monthly average percentage of relative humidity (RH) of the exposure environment observed during the period of study revealed that the average monthly maximum humidity remained high (above 85%) during the period June to September (Fig. S4b†). The annual rainfall varied between 500 to 800 mm in the months of October to December. Major rainfall was recorded during the southwestern monsoon period between June and August and north east monsoon period occurred between October and December (Fig. S4c†). During the second half rainfall was less, wind velocity decreased, and the air pollution level increased. From January, the wind velocity (Fig. S4d†) increased and blew the pollutants away to distant areas, which resulted in a gradual decrease of the air pollutants.3.2 Determination of Cl− and SO2
Seasonal variations of SO2 and Cl− deposited for every month on yearly average in the exposed environment are given in Fig. 1. In general, concentrations of the corrosive agent SO2 began to increase during April, reached a peak during July or August, and then decreased until January or February. But, during the period from March to June, it was more or less stable. In the case of chloride, the variation was small; it was in the range of 65–80 mg m−2 d−1 The SO2 concentration was higher during the autumn. During the first half of the rainy season, a high volume of precipitation improved the air quality. The average concentrations of Cl− and SO2 pollutants during the April 2012 to March 2015 period are summarized in Fig. 1. According to the pollution level as described by ISO 9223, the sites are classified as C5 (ISO 9223-2012).3.3 Surface appearance of the exposed stainless steels
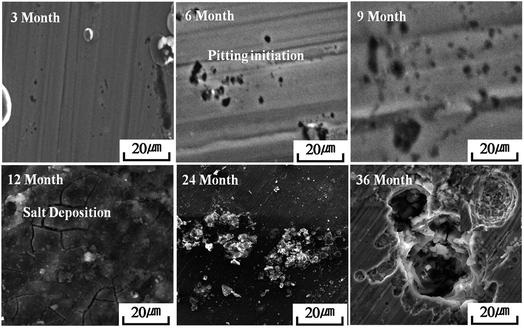
Fig. S5† shows photographic images of the atmospheric-exposed steel specimens from 3 to 36 months of exposure and illustrates the macroscopic morphology of the specimens; the specimens are highly corroded, with localized corrosion (pitting corrosion) products on the exposed surface. Both steels indicate loss in brightness and high surface roughness with yellowish grey colour surface. The surface was uneven because of a larger number of small pits along with corrosion products. The 316L SS surface showed a smaller number of pits when compared to 304 SS. During the initial periods of 3 and 6 months only minimal serious localized corrosion defects were visible on the steel surface. After 9, 12, 24, and 36 months the corrosion defects are much more prominent from the images.3.4 Weight loss and corrosion rate
Weight loss measurements are the most accurate and precise method for determining the corrosion rate. After the exposure of 316L and 304 SS for 3, 6, 9, 12, 24, and 36 months the samples were removed and cleaned according to ASTM G 56-98 standards. Change in weight loss of 316L and 304 SS during their exposure for various durations are given in Fig. 2a. There is drastic variation in the weight loss in the case of 304 SS when compared to 316L SS showing a higher corrosion rate in 304 when compared to 316L SS which further stabilizes over time. The weight losses and corrosion rates were calculated in the atmospheric exposure site; weight losses started from 5 mg to 45 mg after 36 months. During the initial period the corrosion rate was higher for 304 SS when compared to 316L SS and progressively increased up to 9 months. Further, the corrosion rate decreased and reached the lower values after 36 months as are shown Fig. 2b which indicated the formation of passive film and protective rust layers. | ||
| Fig. 2 Change in (a) weight loss and (b) corrosion rate of 316L and 304 SS during exposure in the industrial-marine-urban environment. | ||
The corrosion behavior of SS was maximized during the winter season. This may be attributed to higher moisture content during winter and water holding ability for a longer time on the steel surface when it is cold.25
3.5 SEM analysis
SEM images of surface morphologies of the atmospheric-exposed steel specimens during 3 to 36 months of atmospheric exposure are shown in Fig. 3 and 4. During the initial 3 month exposure, few pits seemed to be formed. After six months, pits with increasing pore diameter and depth were seen, and after 9 months the deposition of salts over pits was easily traceable. EDAX analysis confirmed the presence of Cl and S in the corrosion products at the later stages of exposure. The amount of salt products increased drastically which corresponded with the results obtained from wet candle and sulphation plate analysis. It is commonly known that the atmospheric salt particles could deposit over the exposed steel surface by adsorption phenomena.26,27 Further, these deposited salt particles could enhance the moisture content and SO2 amount over the steel surface during periods of high humidity.27,28 However, high SO2 content leads to formation of a highly stable goethite phase.29 This may be related to the high time of wetness during atmospheric exposure.30 It is obvious that temperature, wind speed, and humidity approached the highest values after 6 months of exposure. This clearly indicates that the stainless steels pits were initiated in a localized manner at critical sites in the early stage of atmospheric environment.31,323.6 AFM analysis
The surface topography of atmospheric-exposed SS samples are depicted in AFM images. Fig. S6 and S7† show the line profile recorded on the samples after 3 to 36 months of exposure in the IMU environment. AFM analysis of exposed SS revealed the fact that passive film on the surface of the specimens was mainly composed of large grains.33–35 Small sized pits were also observed on both the SS samples with grains over their surfaces. From the AFM and 2D line analysis, the development of pit initiation, growth, and passive film formation was observed. The depth and width of pits propagated with exposure time in the atmospheric environment. The atmospheric pollutants made the pits deeper and wider. The formation of pits can be correlated to the change in electrochemical redox behaviour of the austenite and ferrite phases in the stainless steel.36The 2D line analysis offers detailed information about the location of pit initiation and shape of the pits. The pits were initiated after exposure to atmospheric environment. These initial pits identified, as meta stable pits, served as a nucleus for further development during 3 to 6 months exposure. Initially, the metastable pit nucleus was formed very rapidly soon after the dissolution of passive film (oxide film) over stainless steel in an aggressive atmospheric environment.37 However, not all the metastable pits could grow into macro size. The re-passivation effectively inhibits the pit growth, whereas surface defects and dissolution of MnS inclusion enhance the pit size in 304 SS.38 The 2D line profiles of the pitting location during exposure times are shown in Fig. S7† which clearly shows that the pitting was initiated from the 3rd month. The pit depth and size increased very fast as depicted in Fig. S8 and S9.†
3.7 Surface roughness and pit depth analysis
In order to give a clear picture about pit depth and roughness, 2D line analysis was carried out. The depth and width of a pit affects the surface roughness via peak heights and valleys. Further, the high value of surface roughness directly affects and accelerates the corrosion rate by forming a micro-galvanic corrosion couple between the pit depth and width.16,39 Fig. S8 and S9† shows the 2D profile of the atmospheric corrosion exposed SS pitting information obtained from SEM images.Fig. 5a shows the changes in depth of the pits formed after exposure to the atmosphere during different times. Small sized pits spread over the steel surface in the initial period, then pits grow to a larger size with increased the exposure time. Fig. 5a clearly shows that pit depth starts from 10 and 15 μm for 316L SS and 304 SS, respectively. The pit depth and density increases with the exposure time. The maximum pit depth for 316L SS and 304 SS was 39 and 68 μm, respectively. These facts reveal that 316L SS shows better pitting resistance compared to 304 SS. Pit depth analysis shows that roughness values increased by increasing the duration of exposure Fig. 5b.
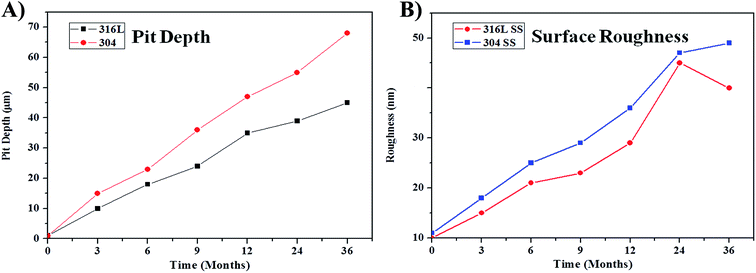 | ||
| Fig. 5 Pit depth and surface roughness of the exposed 316L and 304 SS after exposing in the IMU environment. | ||
3.8 Vickers micro-hardness test
Vickers micro-hardness can be defined as the resistance of the metal to plastic deformation, usually by indentation. It is the property of a metal which gives it ability to resist being permanently deformed when a constant load applied. The greater the hardness of the metal, the greater is the resistance to deformation. The hardness values directly related to the degree of damage to the exposed steel surface. The hardness values mainly depended on pits and deformation of the passive films and solid particles deposition from the atmosphere. Fig. S10† shows measured hardness values of bare and unexposed specimens. Bare 316L SS and 304 SS show higher hardness values than exposed specimens. The hardness value decreases with increasing exposure duration. The decrease in hardness values can be ascribed to the development of micro pits and cracks over the steel surface.3.9 Semi-quantitative analysis of Cl− and SO2 on exposed SS samples
XRF analysis can give reliable quantitative information about chemical composition and concentration of Cl− and SO2 deposits on the SS surface during exposure in an IMU environment. Fig. 6 shows representative results of the XRF intensities of sulphur and chloride on the steel surfaces after 3 to 36 months of exposure. The amounts of Cl− and SO2 deposited on the steel surfaces were much higher during the rainy and winter seasons compared to the dry summer season. XRF analysis showed that the initial exposure could not reveal appreciable deposition of Cl− and SO2 on either SS surface due to their low concentrations. The high amount of corrosive agents' deposition mainly depended on the seasonal characteristics of the exposure season. Higher amounts of corrosive agents were observed at the beginning of the rainy season or at the end of the dry summer season. The XRF results were well in agreement with the determination of corrosive agents measured by the wet candle and sulphation plate methods. After 6 months of exposure a high amount of corrosive agents could be detected in the rainy seasons with high humidity. Also, a drastic increase in elemental composition of Na, Ca, Si, and K was observed on the steel surface. Thus, the rate of moisture and rainfall carrying the corrosive agents were confirmed. | ||
| Fig. 6 XRF analysis of elemental composition of exogenous particle deposited on SS surfaces after 3 years of atmospheric exposure. | ||
But in rain, sulphur content dominates in the form of SO42− anions. Further, EDAX analysis also indicated the dissolution of alloying elements which entered into the corrosion medium along with some of the alloying elements precipitated as corrosion products. In addition, some of the alloying elements like Cr and Ni were enriched in the steel surface. Both the steels showed coloration in varying degrees during atmospheric exposure of the samples. Cr enrichment also occurred in the passive film, thus enhancing corrosion resistance which was also in agreement with the EDAX analysis (Fig. S11†).
3.10 Raman spectroscopy
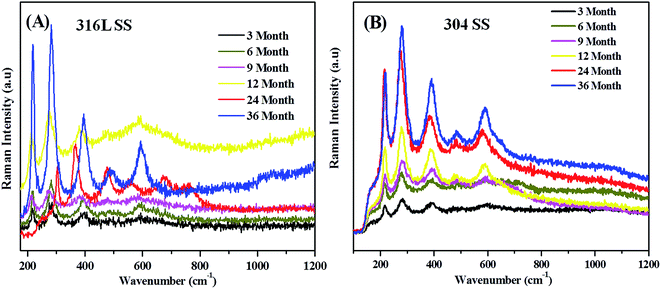
Raman spectra were taken to identify the transition and end products of atmospheric corrosion on 316L and 304 SS specimens during field exposure for 3, 6, 9, 12, 24, and 36 months and tabulated with Raman signatures listed in Table S1.† The relative intensities of Raman signatures obtained for the corrosion products showed relative constituents of the thin rust layers formed during different exposure periods.40 During the initial state the anodic dissolution of steel and the cathodic reduction of oxygen dominated the corrosion processes. Dissolved ferrous ions were hydrolyzed into a deposit of Fe(OH)2 during drying of the aqueous film; Fe(OH)3 was further transformed into an amorphous deposit of FeOOH on the dry surface of the steel. The cathodic reduction was dominated by reduction of FeOOH to Fe3O4. The presence of thick electrolyte on the surface could limit the oxygen reduction rate and in such cases the following reduction supports the oxidation of iron.41| 2FeOOH + Fe2+ + 2e− → 3Fe3O4 |
Magnetite was formed in oxygen depleted systems. With increasing time goethite was converted to either maghemite or haematite. These processes could be witnessed with the Raman signatures obtained for various time periods of exposure. The Raman spectra explained the corrosion processes which is in conformity with the Stratman model.
Fig. 7 and 8 clearly show that during exposure for the first 3 months the corrosion products formed were assigned to lepidocrocite, goethite, and iron oxides at 250, 350, 400, and 600 cm−1, respectively for 316L SS and 300, 400, 500, 600, and 220 cm−1 signatures for goethite and iron oxides, respectively.
 | ||
| Fig. 8 Schematic diagram of the corrosion process and the formation of rust products over the steel surface in IMU environment. | ||
During the next rainy season disappearance of Raman signatures were noted for lepidocrocite, but new peaks appeared at 220 and 500 cm−1 which were assigned to haematite in the case of 316L SS. In the case of 304, peaks corresponding to lepidocrocite appeared at 180 and 240 cm−1 which corresponded to magnetite.42
After the completion of 9 months, only one peak corresponding to goethite appeared at 390 cm−1 and rest of the peaks corresponded to iron oxides in 316L SS. Further, in 304 SS the peak intensities increased with respect to goethite and iron oxides.
The Raman signatures obtained after 12 months for 316L SS and 304 coupons corresponded to iron oxides and goethite formed on the samples. It is interesting to note that after the completion of 2 years, a new peak appeared at 670 cm−1 which corresponded to akaganeite. Also, a peak appeared at 780 cm−1 corresponding to magnetite. The akaganeite peak appeared only under critical concentration of the chloride ion which played a crucial role in formation of akaganeite.43
After 3 years of exposure, the samples revealed formation of stable goethite and iron oxides with sharp peaks. A mixture of goethite in higher amounts and of super paramagnetic maghemite in a lower amount was observed. The formation of goethite can account for the presence of hygroscopic SO2 which lowered the pH of the water, wet the rust, dissolved the corrosion products of lepidocrocite, and promoted its phase transformation into goethite. In general, the Raman intensity depended strongly on parameters like surface roughness and porosity. Variation in Raman intensity for the various iron oxides were attributed to the formation of the corrosion rust produced during the wet and dry cycles, accounting for the surface roughness and porosity.11
3.11 Electrochemical analysis
 | ||
| Fig. 9 EIS curves of SS in 3.5% NaCl solution before and after 3 years of atmospheric exposure: (a and b) Bode plot, (c and d) Bode phase angle curves. | ||
Fig. 9c and d represents the Bode phase angle curves of SS in 3.5% NaCl solution before and after 3 years of atmospheric exposure. Two distinguished maxima could be seen in the Bode phase angle plots; the high frequency maximum increased with time, while the low frequency maximum decreased with time. This indicated that some major changes with the formation of passive film had occurred during the atmospheric corrosion process and showing corrosion resistance of 316L and 304 SS. The formation of protective passive film on the metal surface was mainly influenced by the alloying element composition and corrosive medium in the environment (Cl− and SO2).
The EIS results clearly suggested that the Rp values decreased sharply owing to the formation of pits over the steel surface. Interestingly, there was a sharp increase in breakdown potential with the increase in exposure duration. However, based on discussions of the Raman and XRF results obtained for 12 months exposure, pitting corrosion resistance was enhanced. The significant decreases in impedance values were observed in Bode plots after 6 months of exposure which indicated the occurrence of pitting corrosion. This phenomenon can only be described by passivity breakdown and localized corrosion.
Equivalent circuits comprise Rs-the electrolyte resistance; Rpass and Cpass represent the resistance and capacitance of the passive film, Rct and Cdl corresponds to the charge transfer resistance and double layer capacitance, respectively as shown in Fig. S12.† The Warburg impedance (Wpass) was observed for the dissolution process at breaking potential or pitting potential, which is attributed to the diffusion of corrosive agents (Cl− and SO2) through corrosion products and salt products which get deposited on the pit surface of the steel.44,45 Further, the changes in passive film behaviour can be related to stability structure and electrical conductivity of the passive film.
Fig. S13† clearly shows very high Rp values for the bare SS and it decreases with the increase in exposure time. The higher Rp value indicated good corrosion resistance, and a lower value could indicate the interaction of underlying metal with electrolyte solution accelerating the oxidation and corrosion of the metal. The higher value in Rp could be attributed to passive film stability which is mainly influenced by the presence of N in stainless steels; it could assist faster re-passivation during the pit formation. Very high Rct and Rp values were observed for the bare and initial atmospheric-exposed samples. Rct decreased after 6 months. This phenomenon can be related to pit formation and with the deterioration of passive films over the steel surface. But in the case of 316L SS after 12 months of atmospheric exposure, Rct and Rp values were high and stable when compared to 304 SS. This can be attributed to stable corrosion products of the goethite. 304 SS showed very smaller Rp values after 12 to 36 months which can be related to growth of the pits and formation of new pits as indicated in the SEM analysis. The thin layer of moisture containing the salt particles acted as a diffusion barrier, but it encouraged formation of high porous films and growth of new pits over 304 SS due to the absence of formation of stable rust phases. These results corroborated the existence of pitting corrosion of 304 and 316L SS. This might be due to the mixed controlled process of Rp and Wpass phenomena during the 36 months of long time atmospheric exposure.46 From an electrochemical point of view, easy removal of electrons corresponds to a lower value of Rct, which is the resistance caused by the metal dissolution reaction. Therefore, Rct decreased with increasing surface roughness.
 | ||
| Fig. 10 Potentiodynamic polarization curves of SS in 3.5% NaCl solution before and after 3 years of atmospheric exposure. | ||
Low passive current density was observed for the steels exposed more than 24 months. High current densities were observed for both steels at pitting potentials for bare and initial exposure periods. In general, the breakdown potential and passivation current densities were relatively more sensitive to the passive film composition and exposure duration in the IMU atmosphere.
Variation in anodic current density of the bare and exposed steels from high to low values can be attributed to formation of passive film over the surface. The formed corrosion products can effectively inhibit the anodic dissolution process with increase in the exposure duration.47 In the case of 316L, Mn enhances the pitting corrosion resistance where as in 304 SS MnS dissolution is dominant which results in enhancing the formation of pits.48 The low corrosion current density in 316 can be attributed to enrichment of Cr in passive film formation. Further, the presence of a high amount of SO2 in the environment easily transformed FeOOH into FeSO4 and it enhanced the phase transformation of lepidocrocite into the highly stable goethite form.
The high pitting resistance of the 316L SS can also be attributed to the presence of nitrogen as an alloying element and it can easily form a highly stable phase of iron nitride FeN.49 This can effectively increase the inhibition of the autocatalytic process of pit formation and it increases the chances for pit the healing process.50 Also, the combined effects of alloying elements and environment factors contributed to increasing chances for faster repassivation of damaged passive film.51 Formation of stable and compact passive thin film is clearly indicated because of the passivation processes which was responsible for the high corrosion resistance nature.
Both Eb and ipass increased with increasing goethite content during the initial months of exposure. This revealed the degradation behaviour of passive films and the absence of highly stable corrosion products. The lower ipass can be related to formation of the electrochemical stable phase of goethite in the rust products. It showed higher corrosion resistance and lower passive current of 316L in comparison to 304 steel.50 The passivity region was also broadened with very low passive current density.3 In the case of breakdown potentials, 316L showed high breakdown potentials compared to 304 SS. The cathodic current density curves for bare samples can be related to the reduction of dissolved oxygen present in the corrosive solution.51 The atmospheric-exposed samples can be related to the reduction of corrosion products such as iron oxides and oxyhydroxides. Further, this indicates that the chances of corrosion phenomena are enhanced as the surface roughness values increase (Fig. S14†). High roughness values enhance the interaction between the moisture containing corrosive agents and a metal surface.
4. Conclusions
High alloy steels viz., 316L and 304 SS were field exposed for a period of 3 years (April 2012–March 2015). 304 SS showed more weight loss and corrosion rates compared to 316L SS. The corrosion behavior of SS was found to be maximum during the winter season due to high moisture content. XRF analysis provided reliable quantitative information about the elemental chemical composition of deposited Cl− and SO2 on the SS surfaces. During initial exposure, the XRF analysis did not show the appreciable deposition of Cl− and SO2 on either of the SS surfaces due to the former's low concentration on the steel surface. After 6 months of exposure, the presence of higher amounts of corrosive agents was detected in XRF studies along with drastic increase in the elemental compositions of Na, Ca, Si, and K. AFM analysis of exposed 316L and 304 SS confirmed the presence of a passive film comprised of small and large grains of different sizes. The measured roughness values were found to be around 20 to 40 nm. The formation of stable goethite and iron oxides was confirmed by sharp peaks observed in Raman spectra. We found a mixture of goethite and maghemite, in which goethite was in a higher amount than the maghemite. Polarization studies confirmed higher corrosion resistance through lower passive current density for 316L in comparison with 304 steel. The higher Rp values in EIS spectra confirmed the enhanced corrosion resistance of exposed samples. From results obtained in the present investigation, it was inferred that 316L SS exhibited better corrosion resistance in a highly severe atmospheric environment.References
- T. J. Mesquita, E. Chauveau, M. Mantel, N. Bouvier and D. Koschel, Corros. Sci., 2014, 81, 152–161 CrossRef CAS.
- C. Bitondo, A. Bossio, T. Monetta, M. Curioni and F. Bellucci, Corros. Sci., 2014, 87, 6–10 CrossRef CAS.
- J. H. Qiu, Surf. Interface Anal., 2002, 33, 830–833 CrossRef CAS.
- R. E. Melchers, Corros. Sci., 2013, 68, 186–194 CrossRef CAS.
- T. Laitinen, Corros. Sci., 2000, 42, 421–441 CrossRef CAS.
- Y. Tsutsumi, A. Nishikata and T. Tsuru, Corros. Sci., 2007, 49, 1394–1407 CrossRef CAS.
- S. Atashin, A. S. Toloei and M. Pakshir, J. Mater. Eng. Perform., 2013, 22, 2038–2047 CrossRef CAS.
- S. Atashin, M. Pakshir and A. Yazdani, Mater. Des., 2011, 32, 1315–1324 CrossRef CAS.
- W. Kuang, X. Wu and E.-H. Han, Corros. Sci., 2010, 52, 4081–4087 CrossRef CAS.
- B. Stellwag, Corros. Sci., 1998, 40, 337–370 CrossRef CAS.
- J. Maslar, W. Hurst, W. Bowers Jr and J. Hendricks, Corrosion, 2002, 58, 739–747 CrossRef CAS.
- C. Melendres, M. Pankuch, Y. Li and R. Knight, Electrochim. Acta, 1992, 37, 2747–2754 CrossRef CAS.
- A. Nishikata, Y. Yamashita, H. Katayama, T. Tsuru, K. Tanabe and H. Mabuchi, Corros. Sci., 1995, 37, 2059–2069 CrossRef CAS.
- M. B. Leban, Č. Mikyška, T. Kosec, B. Markoli and J. Kovač, J. Mater. Eng. Perform., 2014, 23, 1695–1702 CrossRef CAS.
- W. Lv, C. Pan, W. Su, Z. Wang, S. Liu and C. Wang, J. Mater. Eng. Perform., 2015, 24, 2597–2604 CrossRef CAS.
- D. Wallinder, I. O. Wallinder and C. Leygraf, Corrosion, 2003, 59, 220–227 CrossRef CAS.
- C. F. Dong, H. Luo, K. Xiao, Y. Ding, P. H. Li and X. G. Li, Anal. Lett., 2013, 46, 142–155 CrossRef.
- S. R. Street, N. Mi, A. J. M. C. Cook, H. B. Mohammed-Ali, L. Guo, T. Rayment and A. J. Davenport, Faraday Discuss., 2015, 180, 251–265 RSC.
- M. Natesan, S. Palraj, G. Venkatachari and N. Palaniswamy, Corrosion, 2006, 62, 883–891 CrossRef CAS.
- M. Natesan, S. Selvaraj, T. Manickam and G. Venkatachari, Sci. Technol. Adv. Mater., 2008, 9, 045002 CrossRef.
- D. Singh, S. Yadav and J. K. Saha, Corros. Sci., 2008, 50, 93–110 CrossRef CAS.
- P. Dhaiveegan, N. Elangovan, T. Nishimura and N. Rajendran, Electroanalysis, 2014, 26, 2419–2428 CrossRef CAS.
- P. Dhaiveegan, N. Elangovan, T. Nishimura and N. Rajendran, Mater. Trans., 2016, 57, 148–155 CrossRef.
- ASTM G 50-76, Standard practice for conducting atmospheric corrosion tests on metals, 1997, vol. 11.02, pp. 1–5 Search PubMed.
- T. T. N. Lan, R. Nishimura, Y. Tsujino, K. Imamura, M. Warashina, N. T. Hoang and Y. Maeda, Anal. Sci., 2004, 20, 213–217 CrossRef CAS PubMed.
- P. Mohan, M. Natesan, M. Sundaram and K. Balakrishnan, Bull. Electrochem., 1996, 12, 91–92 CrossRef CAS.
- P. Jin, W. Zhang, Q. Wang, X. Yang, S. Sun, X. Fan and B. Li, Corros. Sci., 2014, 89, 268–274 CrossRef CAS.
- T. E. Graedel and C. Leygraf, Electrochem. Soc. Interface, 2001, 10(4), 24–30 CAS.
- Y. Martín-Regueira, O. Ledea, F. Corvo and C. Lariot, Corros. Eng., Sci. Technol., 2011, 46, 624–633 CrossRef.
- D. Singh, S. Yadav and J. K. Saha, Corros. Sci., 2008, 50, 93–110 CrossRef CAS.
- X. Cao, H. Deng, W. Lan and P. Cao, Anti-Corros. Methods Mater., 2013, 60, 199–205 CrossRef CAS.
- (a) E. Schindelholz, R. G. Kelly, I. S. Cole, W. D. Ganther and T. H. Muster, Corros. Sci., 2013, 67, 233–241 CrossRef CAS; (b) E. Schindelholz, B. E. Risteen and R. G. Kelly, J. Electrochem. Soc., 2014, 161, C450–C459 CrossRef CAS.
- A. Burkert, H. Klapper and J. Lehmann, Mater. Corros., 2013, 64, 675–682 CrossRef CAS.
- K. Umemura, N. Nishida, M. Hara, H. Sasabe and W. Knoll, J. Electroanal. Chem., 1997, 438, 207–211 CrossRef CAS.
- H. Yang, P. Pi, Z.-Q. Cai, X. Wen, X. Wang, J. Cheng and Z.-r. Yang, Appl. Surf. Sci., 2010, 256, 4095–4102 CrossRef CAS.
- P. J. Antony, S. Chongdar, P. Kumar and R. Raman, Electrochim. Acta, 2007, 52, 3985–3994 CrossRef CAS.
- S. Fréchard, F. Martin, C. Clément and J. Cousty, Mater. Sci. Eng., A, 2006, 418, 312–319 CrossRef.
- L. Liu, Y. Li and F. Wang, Electrochim. Acta, 2008, 54, 768–780 CrossRef CAS.
- I. Reynaud-Laporte, M. Vayer, J.-P. Kauffmann and R. Erre, An Electrochemical-AFM Study of the Initiation of the Pitting Corrosion of a Martensitic Stainless Steel, 1997 Search PubMed.
- S. M. Cambier, D. Verreault and G. S. Frankel, Corrosion, 2014, 70, 1219–1229 CrossRef.
- Q. W. Knapp and J. C. Wren, Electrochim. Acta, 2012, 80, 90–99 CrossRef CAS.
- D. Thierry, D. Persson, C. Leygraf, N. Boucherit and A. Hugot-le Goff, Corros. Sci., 1991, 32, 273–284 CrossRef CAS.
- T. Nishimura, H. Katayama, K. Noda and T. Kodama, Corrosion, 2000, 56, 935–941 CrossRef CAS.
- G. Herting, I. Odnevall Wallinder and C. Leygraf, Corros. Sci., 2006, 48, 2120–2132 CrossRef CAS.
- J. L. Dawson and M. G. S. Ferreira, Corros. Sci., 1986, 26, 1027–1040 CrossRef CAS.
- L. Hao, S. Zhang, J. Dong and W. Ke, Corros. Sci., 2011, 53, 4187–4192 CrossRef CAS.
- S. Nagarajan, S. Tamilselvi and N. Rajendran, Mater. Corros., 2007, 58, 33–38 CrossRef CAS.
- S. Nagarajan, M. Karthega and N. Rajendran, J. Appl. Electrochem., 2007, 37, 195–201 CrossRef CAS.
- R. Qvarfort, Corros. Sci., 1998, 40, 215–223 CrossRef CAS.
- L. Freire, M. A. Catarino, M. I. Godinho, M. J. Ferreira, M. G. S. Ferreira, A. M. P. Simões and M. F. Montemor, Cem. Concr. Compos., 2012, 34, 1075–1081 CrossRef CAS.
- L. Yao, J. Liu, S. Li and M. Yu, Corros. Sci., 2014, 80, 12–18 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04015b |
| This journal is © The Royal Society of Chemistry 2016 |