Copper(ii)-catalyzed remote sulfonylation of aminoquinolines with sodium sulfinates via radical coupling†
Abstract
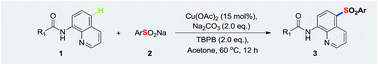
The efficient remote sulfonylation of N-(quinolin-8-yl)benzamide derivatives at the C5 position has been well developed. The reaction generates environmentally benign byproducts by utilizing stable and safe sodium sulfinates as sulfide sources. A series of N-(5-(phenylsulfonyl)quinolin-8-yl)benzamide derivatives were successfully obtained in moderate to high yields. In particular, they are less unpleasantly odorous and more environmentally friendly than previous means.
- This article is part of the themed collection: Editors’ collection: Catalytic Organic Transformations

 Please wait while we load your content...
Please wait while we load your content...