Wall-loaded Pt/TiO2/Ti catalyst and its application in ammonia oxidation reaction in microchannel reactor
Wei Jiang*,
Jiaping Qiu,
Wei Qiu,
Shaojun Yuan,
Houfang Lu and
Bin Liang
Multi-Phases Mass Transfer and Reaction Engineering Laboratory, College of Chemical Engineering, Sichuan University, Wangjiang campus, No. 24 South Section 1, Yihuan Road, Chengdu 610065, China. E-mail: weijiang@scu.edu.cn; Fax: +86-28-85460556; Tel: +86-28-85990133
First published on 7th March 2016
Abstract
A microchannel reactor is a promising reactor type for industrial applications. However, loading heterogeneous catalysts in the microchannels is troublesome because of the high pressure drop and serious fluid flow abrasion. In this research, a home-made microchannel reactor was designed by using a wall-loaded Pt/TiO2/Ti catalyst as the microchannel wall. This wall-loaded Pt/TiO2/Ti catalyst was prepared by anodizing a Ti foil and photodepositing Pt on the anodic surface layer. The foil was then assembled as a side wall of the microchannel. Ammonia oxidation was used as the probe reaction for evaluating the performance of this microchannel reactor by using the wall-loaded catalyst. Simulation and experimental results show that 100% ammonia conversion can be obtained under the following conditions: temperature, 300 °C; gaseous hourly space velocity, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; catalyst loading percentage, 85% of the total channel area; and ammonia to oxygen volume ratio, 1
000 h−1; catalyst loading percentage, 85% of the total channel area; and ammonia to oxygen volume ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, in the absence of nitrogen, with a microchannel of length 80 mm, width 1.0 mm, and height 0.3 mm. Pressure drop in the microchannel is as small as 1.18 kPa, and temperature distribution is relatively uniform. Notably, NOx selectivity is significantly improved to become 88.32% at 300 °C. Simulations confirm that the selectivity showed such a high improvement owing to the short residence time of NOx because of the unique blind-hole structure of TiO2 nanotubes and the resulting large mass transfer resistance. The operation of this reactor with the wall-loaded Pt/TiO2/Ti catalyst is stable without any remarkable performance decay even after 360 h of operation. Hence, the microchannel reactor with a wall-loaded catalyst on the anodic metal is an attractive prospect for large-scale applications of rapid gas-to-solid exothermic reactions.
13, in the absence of nitrogen, with a microchannel of length 80 mm, width 1.0 mm, and height 0.3 mm. Pressure drop in the microchannel is as small as 1.18 kPa, and temperature distribution is relatively uniform. Notably, NOx selectivity is significantly improved to become 88.32% at 300 °C. Simulations confirm that the selectivity showed such a high improvement owing to the short residence time of NOx because of the unique blind-hole structure of TiO2 nanotubes and the resulting large mass transfer resistance. The operation of this reactor with the wall-loaded Pt/TiO2/Ti catalyst is stable without any remarkable performance decay even after 360 h of operation. Hence, the microchannel reactor with a wall-loaded catalyst on the anodic metal is an attractive prospect for large-scale applications of rapid gas-to-solid exothermic reactions.
1. Introduction
Microreactor technology has become synonymous with microfabricated systems comprising miniaturized continuous flow systems, especially channels, with features that are as small as 10 nm to those as big as several hundred micrometers. In these channels, fluids flow continuously and chemical reactions occur.1–3 Process intensification based on microreactors has been realized in chemical engineering in order to reduce capital and energy costs and the negative environmental impact by decreasing the size of the chemical plant.4 Owing to their small size and high surface to volume ratio (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m2 m−3), the use of microreactors for chemical production affords a number of advantages such as high mass and heat transfer, increased safety, easy scale-up, good controllability, and better conversion and selectivity.3 Different kinds of materials are used for microreactor design, such as stainless steel, glass, silicon, and polymers.5,6 Because homogeneous catalysts show excellent activity and selectivity, they are usually used for carrying out most of the reactions in microreactors. However, the inherent defects of homogeneous catalysts such as the difficulty in removing and reusing them and the possibility of product contamination by the catalyst metal significantly decrease their attractiveness. Hence, heterogeneous catalysts are used for microchannel reactors because they do not have the above disadvantages.6,7
000 m2 m−3), the use of microreactors for chemical production affords a number of advantages such as high mass and heat transfer, increased safety, easy scale-up, good controllability, and better conversion and selectivity.3 Different kinds of materials are used for microreactor design, such as stainless steel, glass, silicon, and polymers.5,6 Because homogeneous catalysts show excellent activity and selectivity, they are usually used for carrying out most of the reactions in microreactors. However, the inherent defects of homogeneous catalysts such as the difficulty in removing and reusing them and the possibility of product contamination by the catalyst metal significantly decrease their attractiveness. Hence, heterogeneous catalysts are used for microchannel reactors because they do not have the above disadvantages.6,7
Two common strategies are used for employing heterogeneous catalysts in microreactors. One is to load the active species on small solid supports such as SiO2, magnetic nanoparticles, and molecular sieves, which coflows with the reactant and is filtered after reaction completion. The other is to load the active species on the wall of microchannels so that there is no need to detach heterogeneous catalysts, which saves the catalyst consumption and cost. The latter is more attractive for further study and possible large-scale industrial applications than the former because of easy catalyst recovery and reusage, absence of any abrasion of microchannels, and low flow resistance.
Depending on the properties of the surface and the catalyst deposited, different methods such as sol–gel, anodic oxidation, and thermal treatment can be used to deposit a catalyst layer on a surface.8–11 Among them, the anodic oxidation method is generally used to obtain a porous layer on the surface of Si and Al foil to load active species.12–15 This method is either used as a pretreatment before using another coating method, or as a way to obtain a thin porous layer that can be directly impregnated.8,16
Because of the high thermal conductivity of the metal substrate, regular subtle nanostructure of anodic metal oxides, and high adhesion between the metal substrate and the metal oxide layer owing to in situ growth, the metal oxide/metal compound structure has wide application prospects in the design and fabrication of microreactors. However, most of the reports focus on the utilization of anodic alumina and silica.8,15 The use of other anodic materials for microreactor has been rarely reported.
Anodization process of valve metals, including Ti, Zr, Fe, and Cu, has been extensively investigated. Various regular nanostructures, including nanotube, nanorod, and nanowire arrays, have been prepared by anodization and used for energy storage, biochemistry, and environmental protection.17–24 These anodic metal materials can be used with microreactors because of the similar merits of anodic alumina, and existing applications of their oxides as catalyst support.
There is another possible advantage of using anodic valve metals in microreactors. Most of the valve metal oxides, especially TiO2, show considerably high photocatalytic performance,25–28 and can deposit noble metals such as Ag, Au, Pt, and Pd by photoreduction.29–32 Theoretically, noble metals loaded this way are deposited in single-atom layer dispersion on photocatalyst supports, which improves the catalytic performance and decreases the loading amount.
In this research, a microchannel reactor is designed and fabricated by preparing a wall-loaded catalyst on an anodic Ti foil. A flat Ti foil was anodized as the support by using a regular TiO2 nanotube array for loading Pt by photoreduction. Because ammonia oxidization, which is a highly exothermic reaction and is used in the nitric acid industry, has been well understood and possess a series of reactions in process for selectivity studying,33 it was selected as the probe reaction to evaluate the performance of this home-made Ti-based microreactor with the wall-loaded Pt/TiO2/Ti catalyst.25 The operation parameters of this microreactor have also been optimized, and its advantages have been reported. Further application of such wall-loaded Pt/TiO2/Ti catalyst on other reactions such as hydrogenation of benzene and purification of automobile tail gas will be attempted in future.
2. Experiment
2.1. Materials
Different Ti foils (purity 99.9%) of thickness 0.1, 0.2, 0.3 and 0.5 mm were purchased from Hebei Qingyuan Metal Materials Co., Ltd. Ammonia (purity 99.9%) was supplied by Chengdu Dongfeng Industrial Gas Business Department and nitrogen and oxygen (purity 99.9%) were supplied by Chengdu Shuangliu Industrial Gas Business Department. Potassium hexachloroplatinate (K2PtCl6; purity 99%) was purchased from Beijing HWRK Chem. Co., Ltd. Other analytically pure chemicals, including acetone, ethanol, hydrofluoric acid, phosphoric acid, sodium hydroxide, and formic acid, were purchased from Chengdu Kelong Chemical Reagent Co., Ltd., and used directly without further purification.2.2. Anodization of Ti foil
Pure Ti foils of different thicknesses were cut into small rectangular sheets of dimensions 20 × 40 mm. They were polished mechanically with an abrasive paper and were then ultrasonically cleaned in ethanol, acetone, and deionized water before anodization. After drying under a N2 flow, the pretreated Ti foil was used as the anode, while a Pt foil (20 × 20 × 0.2 mm) was used as the cathode. The electrolyte was prepared by adding 1.5 mL HF and 23.7 mL H3PO4 into 300 mL water; the resulting solution was then transferred into an electrolytic cell at a constant temperature of 30 °C. Under a constant voltage of 20 V, the Ti foil was anodized for about 120 min to construct the anodic layer. After anodization, the sample was rinsed immediately with deionized water for 3 min. Finally, the anodized Ti foil was dried overnight in an oven at 80 °C and calcined in a muffle furnace at 450 °C under a N2 atmosphere for about 2 h.2.3. Photocatalytic deposition of Pt on anodic Ti foil
The as-prepared Ti foil after anodization was placed on the bottom of a flat glass culture dish. One mL K2PtCl6 solution (0.0036 mol L−1) and 10 mL deionized water was dropped into the culture dish, and a few drops of methanol were added as a sacrificial reagent to capture the photogenerated holes. Hereafter, the culture dish was placed horizontally under a 500 W UV lamp and was illuminated for about 30 min with light from the vertical direction. The color of the anodized titanium foil changed from light yellow to black. Then, the as-prepared sample was dried overnight in an oven at 80 °C.Three other catalyst samples—fine Pt/TiO2 particles, wall-loaded Pt/TiO2 on a calcined Ti foil by photodeposition, and wall-loaded Pt/TiO2 on an anodized Ti foil by impregnation—were also prepared for comparison. The fine TiO2 particle support was prepared by a sol–gel process, and was screened with a 500 mesh sample sieve. The calcined Ti foil was prepared by annealing a pure Ti foil in a muffle furnace at 450 °C under air atmosphere for 6 h. The photocatalytic deposition process of Pt on fine particles as well as that on the calcined Ti foil was similar to the photocatalytic deposition procedure described above. The third support was prepared as described in Section 2.2, but the photocatalytic deposition step of Pt loading was replaced with the impregnation step by immersing the anodic Ti sample into the K2PtCl6 solution for about 2 h without any light irradiation.
2.4. Characterization of the as-prepared sample
Surface morphology and composition of the fresh and used Pt/TiO2/Ti samples were conducted by using scanning electron microscopy (JSM-7500F). The surface element components and valence were determined on a VG ESCALAB MKII XPS system with an Al Kα source and a charge neutralizer. XRD analysis was conducted by glancing angle X-ray diffraction (GAXRD; DX-2007 SSC) with a Cu Kα 40 kV/30 mA X-ray source.2.5. Design and operation of microreactor
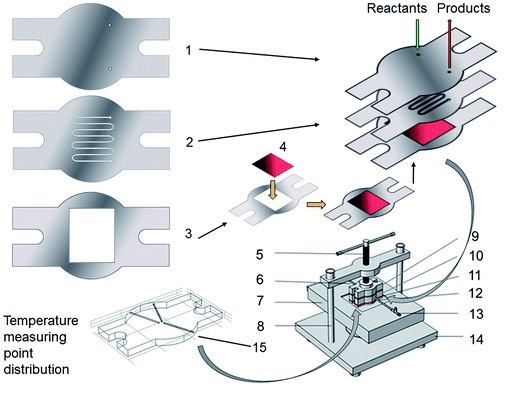
A stacked sandwich-type microreactor was designed to evaluate the performance of the Pt/TiO2/Ti wall-loaded catalyst. Three plates made of the treated Ti foil: catalyst plate, microchannel plate, and cover plate (marked as plates 1, 2, and 3 in Fig. 1, respectively), were stacked to form a microchannel unit. The use of the treated inert Ti foil after high-temperature oxidation at 700 °C can help in effectively avoiding the disturbance resulting from the reactor materials.A rectangular hole having the same dimension as that of the as-prepared Pt/TiO2/Ti sample was scratched out on the catalyst plate shown in Fig. 1. The catalyst sample (shown in red and marked as 4 in Fig. 1) can be tightly placed into the hole. This led to the formation of a catalyst plate with a wall-loaded catalyst on one side of the microchannel.
The microchannel plate with a channel was the second layer that covers the catalyst plate and forms a flow path for reactants. The shape and size of the microchannels were optimized by finite element modeling (FEM) simulation and were fabricated by using a wire-cut electrical discharge machine. The actual dimensions of the microchannel in this research were as follows: height, 0.1–1.0 mm; width, 0.2–1.0 mm; and length, 10–120 mm.
The third layer, the cover plate, was laid over the microchannel plate, forming the other wall of the microchannel. This wall was inert for ammonia oxidation. Two holes of diameter 1 mm were drilled on the cover plate as the input and output of the microchannel for the reactant and product streams, respectively, and were accurately positioned to the starting and terminal points of the microchannel.
The three plates formed the microchannel unit, which was assembled into the home-made microreactor equipment. An electric heating plate, marked as 7, was fixed on the bottom as the heat source. A thermocouple plate made of a 5 mm-thick Cu foil (shown in red and marked as 12) was installed between the heat plate and the microchannel unit for controlling and monitoring the temperature of the reacting unit. The distribution of the temperature measuring point is shown in 15 (inserted graph in Fig. 1).
An 8 mm-thick stainless steel plate, marked as 10, was inserted above the microchannel unit; this plate was drilled and connected to both the gas inlet and outlet pipes. The top stainless steel plate, marked as 9, transfers the pressure from the screw compressive bar by tightly adpressing all components of the microreactor and prevents any possible gas leakage.
The complete flow chart of the experimental setup used for evaluating the microreactor is shown in Fig. 2. The gaseous reactants, NH3 and O2, released from cylinders 1 and 2 were fed into the microreactor, and were then monitored and controlled by using external mass flow meters. The ammonia oxidation process was performed in the microreactor. To prevent any gas leakage, the total gas flow rate was controlled to be less than 60 mL min−1. The exhaust of the microreactor was continuously analyzed with a gas chromatograph (GC) equipped with a GDX column and a 5A molecular sieve column (GC-200, Chongqing Chuanyi Analyzer Co. Ltd.) online. A temperature controller, marked as 7, was used to control and detect the temperature of the microreactor. In the reaction process, the thermal behavior, pressure drop, NH3 conversion, and NOx selectivity were all determined simultaneously.
2.6. Simulation and optimization of microchannels
The microreactor unit was modeled, meshed, and solved by FEM to determine and optimize the geometry shape and size of the microchannels. A typical 3D model of the microreactor for simulation was developed and is shown in Fig. 3. | ||
| Fig. 3 The 3D model constructed for FEM simulation (A) the 3D model of microchannel; (B) the simulation of ammonia oxidation reaction in microchannel. | ||
As is well known, the small dimensions of the microreactor systems usually implied a strong influence of diffusion on the transport process because of a small Reynolds number. The gas flow pattern in this flattening microchannel is laminar since the calculated Reynolds number is less than 1. Therefore, diffusion can be reasonably regarded as the controlling factor of the mass transfer in the microchannel, which can be described by the diffusion equation shown in eqn (1):
| ∇(−Di∇ci + ciu) = 0 | (1) |
The continuity equation of the gas flow can be described by eqn (2):
| ∇(pu) = 0 | (2) |
The classical continuum description was also applicable because the characteristic dimension of the microchannels is still sufficiently large compared to the gas mean free path. Therefore, conservation equations (eqn (3)) can be used to determine the reactive flows in this model:34
 | (3) |
Ammonia oxidation process on Pt are relatively well understood, and the process kinetics, as proposed by Pignet and Schmidt, comprises five reaction steps as shown below:27
| 4NH3 + 5O2 → 4NO + 6H2O; ΔH = −904 kJ | (R1) |
| 4NH3 + 6NO → 5N2 + 6H2O; ΔH = −1816 kJ | (R2) |
| 2NO → N2 + O2; ΔH = −90 kJ | (R3) |
| 2NH3 → N2 + 3H2; ΔH = 98 kJ | (R4) |
| 2H2 + O2→ 2H2O; ΔH = −484 kJ | (R5) |
However, reaction (R1) produced NO and reactions (R2) and (R4) represented the series processes leading to the production of N2 through a NO intermediate. Reactions (R1) and (R2) dominate at low temperatures, whereas (R3), (R4), and (R5) are significant only at very high temperatures (>900 °C).35 Because the experimental operating temperature in this research ranged only from 200 to 400 °C, reactions (R1) and (R2) were significant for this simulation, and the other three should be negligible. Therefore, the reaction rates employed can be expressed in terms of Langmuir–Hinshelwood kinetics as follows:
 | (4) |
 | (5) |
3. Results and discussion
3.1. Characteristics of the wall-loaded Pt/TiO2/Ti catalyst
The as-prepared wall-loaded Pt/TiO2/Ti catalyst before and after being used is characterized to determine its components and surface morphology. The nanotube array structure of the catalyst can be clearly observed (Fig. 4A–D). These nanotubes have diameters of 80–100 nm and are placed closely together in an orderly arrangement. Nanoparticles of dimensions 20–100 nm are present on both the top and inside the nanotubes. In particular, many nanoparticles (marked by a red circle in Fig. 4B and D) in the catalyst interiors can be seen to adhere on the nanotube walls. This morphology of the catalyst does not show any obvious change both before and after the reaction, thus confirming the stability of the catalyst structure.EDS analysis results of this structured surface affirm that the nanoparticles dispersed on the surface of TiO2 nanotubes are those of Pt. The atom percent of the Pt nanoparticles before and after the reaction are 1.63% and 1.61%, respectively. This almost constant Pt content also confirms the stability of the Pt/TiO2/Ti catalyst. The slight decrease in the Pt content is perhaps owing to the detachment caused by the erosion of the gas flow because some nanoparticles grow on the top of the nanotubes instead of inside them.
In Fig. 4E, the XRD patterns of fresh and used Pt/TiO2/Ti catalysts in the ammonia oxidation reaction are compared. It can be seen that the anodic TiO2 nanotube layer on the Ti foil before and after the reaction both are made of the anatase phase and does not show any significant crystal phase transformation. However, the characteristic peaks of pure Pt are observed after the ammonia oxidization reaction. It can be inferred that the amorphous Pt photodeposited on the TiO2 nanotubes, which cannot be observed in the XRD pattern as fresh samples, is transformed into crystalline Pt after participating in the catalytic ammonia oxidization reaction. Such a phase transformation can be ascribed to the participation of Pt as the catalyst for ammonia oxidization.
XPS analysis of the fresh and used Pt/TiO2/Ti catalysts also confirms the deduction based on the XRD results (Fig. 4F and G). No significant change in the element content is observed in the full XPS spectrum of the Pt/TiO2/Ti catalyst. However, the relative contents of Pt0 and Pt4+ increase sharply from 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 to 87
62 to 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 before and after the reaction, respectively. High ratio of Pt4+ in the fresh sample can be attributed to the impregnation and adsorption of the K2PtCl6 solute on the porous nanotube structure, especially on the bottom of the TiO2 nanotubes where UV light cannot reach and irradiate directly. However, after ammonia oxidization, K2PtCl6 salts can be decomposed and reduced, which increases the content of Pt0 and improves the degree of Pt crystallinity. Fortunately, such transformation of Pt active species does not influence the dispersion and particle size of Pt nanoparticles, which has been confirmed by the SEM results. Thus, it can be expected that the usage of the Pt/TiO2/Ti sample does not causes any significant decay in its catalytic performance.
13 before and after the reaction, respectively. High ratio of Pt4+ in the fresh sample can be attributed to the impregnation and adsorption of the K2PtCl6 solute on the porous nanotube structure, especially on the bottom of the TiO2 nanotubes where UV light cannot reach and irradiate directly. However, after ammonia oxidization, K2PtCl6 salts can be decomposed and reduced, which increases the content of Pt0 and improves the degree of Pt crystallinity. Fortunately, such transformation of Pt active species does not influence the dispersion and particle size of Pt nanoparticles, which has been confirmed by the SEM results. Thus, it can be expected that the usage of the Pt/TiO2/Ti sample does not causes any significant decay in its catalytic performance.
3.2. Effect of channel length on ammonia conversion
The as-prepared Pt/TiO2/Ti catalyst is assembled into the home-made microdevice shown in Fig. 2. In this research, the shape of the channel cross-section is set as a rectangle beforehand because the catalyst sample was flat. Only the bottom wall of the microchannels is made of Pt/TiO2/Ti, which is beneficial to understand the influence of the flat wall-loaded catalyst and to investigate the effect of mass transfer on the reaction. The top wall and the other two flank walls of the rectangular microchannels are made of the inert TiO2 layer, which was obtained by thermal treating of the pure Ti foil in atmosphere.Owing to the difficulty of mechanical processing, only FEM analysis is conducted to determine the influence of the microchannel length. Three typical reaction temperatures, 873, 945, and 965 K, were adopted to determine the optimal length of the channels. The width of the microchannel was fixed as 1.0 mm and its height as 0.3 mm. The ammonia to oxygen molar ratio was maintained at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, and the gaseous hourly space velocity (GHSV) was 35
13, and the gaseous hourly space velocity (GHSV) was 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. Here, the concept of GHSV in a microchannel reactor with a wall-loaded catalyst is defined as follows:
000 h−1. Here, the concept of GHSV in a microchannel reactor with a wall-loaded catalyst is defined as follows:
| GHSV = volume flow/(catalyst loaded area × height of microchannel) |
The straight microchannels with different lengths were simulated, and the results are shown in Fig. 5A. The simulation results confirm that the total length of the microchannels should be larger than 80 mm for the complete conversion of ammonia. Therefore, the length of the microchannel for microreactor fabrication is kept at 80 mm.
 | ||
| Fig. 5 The effect of geometric shape of microchannel by FEM simulation (A) effect of microchannel length; (B) effect of microchannel corner. | ||
However, an 80 mm microchannel cannot completely expand on the microchannel plate because the dimension of the as-prepared Pt/TiO2/Ti catalyst is only 20 × 40 mm. If the microchannel is machined as a straight channel, its length will exceed the catalyst zone. Therefore, folding of the microchannel is unavoidable in order to achieve an overlong microchannel with the desired length within the limited space. The effect of the corner shape for connecting the folded microchannels is simulated to determine the optimal microchannel. The results are shown in Fig. 5B, which affirms that the semicircle corner, 1#, has the largest ammonia conversion. Thus, the optimal geometric design of the microchannel is the one shown in Fig. 3.
3.3. Effect of channel dimension on ammonia conversion
After determining the length of the microchannel, its height and width are optimized and determined by simultaneously performing simulations and experiments. Ammonia conversion and a pressure drop in the microchannel are used as the indexes for optimization.For a stack design, it is easy to adjust the microchannel height by changing the number of the stacked microchannel plates. In the present work, the microchannel height for optimization ranges from 0.1 to 1.0 mm. The length and width of the microchannel are kept at 80 and 1.0 mm, respectively. The other operation parameters were not changed.
The simulations and experimental results of the ammonia reaction show that the experimental value of conversion is almost in agreement with that of simulation (Fig. 6A). Ammonia conversion decreased sharply on increasing the microchannel height at the same operation temperature. If the microchannel is taller than 0.3 mm, ammonia cannot be completely oxidized even if the temperature reaches 400 °C. This can be explained by the effect of diffusion distance since ammonia oxidization reaction, a typical rapid exothermic reaction with a very large reaction equilibrium constant (Kp) of 8.265 × 1026 at 400 °C is a kinetically controlled reaction. The diffusion distance of the reactants from the bulk gas phase to one side of the wall-loaded catalyst in a microchannel increased significantly, thus remarkably decreasing the ammonia conversion.
Pressure drop inside the microchannel is experimentally determined with a simple U-tube differential gauge. Not surprisingly, the pressure drop in the microchannel reactor shown in Fig. 6B decreases sharply from 5.80 to 0.23 kPa on increasing the microchannel height from 0.1 to 0.9 mm at an operation temperature of 400 °C. On further increasing the operation temperature, the pressure drop in the same microchannel also increases remarkably. Notably, the pressure drop in the 0.3 mm microchannel is only about a third to a quarter of that in the 0.1 mm microchannel. Therefore, the optimum height of the microchannel is determined as 0.3 mm for excluding the effect of external diffusion in the height direction and decreasing the pressure drop on the basis of the maximum output of microreactor.
The effect of increasing the microchannel width from 0.2 to 1.0 mm on ammonia conversion and pressure drop was also investigated (Fig. 6C and D); no significant effect of the channel width was observed on ammonia conversion. However, as expected, the pressure drop declines sharply upon increasing the width. Thus, the maximum simulated value of the microchannel width is selected from the viewpoint of easy manufacturing, low resistance, and high production capacity. Then, the actual cross section of the microchannels is built as a flat rectangular tube with a width of 1.0 mm and height of 0.3 mm.
Another important index, selectivity of NOx, is not taken into account for optimizing the microchannel shape because its value does not show any obvious dependence on the change in the height and width of the microchannel. This is easy to understand because the same wall-loaded catalyst was employed.
3.4. Operation parameter optimization of the wall-loaded microreactor
Since the optimal geometrical parameters of the microreactor for evaluating the wall-loaded catalyst have been determined, the operation parameters of this microreactor, including the catalyst dosage, space velocity, NH3/O2 ratio of feed gas, and nitrogen concentration in feeding, should also be investigated.The control of the catalyst dosage used in the microchannel is realized by adjusting the actual loading area of Pt on the anodized Ti foils. A certain area of the anodic Ti foil was sealed with a tape in the photodeposition process. Then, the covered surface of the anodic Ti foil without the Pt catalyst loading can be regarded as an inert area for carrying out the reaction. When the Pt-loaded area was less than 85% of the total area of the microchannel bottom (i.e., 80 mm2), the ammonia conversion started to decrease (Fig. 7A). The declining amplitude is proportional to the decrease in the catalyst-loaded area. However, on increasing the reaction temperature from 250 °C to 400 °C, the ammonia conversion with different catalyst dosage gradually increased, and finally converged to about 100%. This status means that the activity of the wall-loaded catalyst sharply increases on increasing the reaction temperature. In this microreactor, the actual amount of the noble metal used can be greatly reduced by increasing the reaction temperature. However, in this research, the catalyst dosage is still kept at 85% for maintaining the operation temperature lower than 400 °C.
The GHSV of the NH3/O2 gas feed is changed to determine its effect on ammonia conversion. The value of GHSV in the present experiment ranged from 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 50
000 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. The maximum setting value of GHSV, 50
000 h−1. The maximum setting value of GHSV, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, is determined by the safe operation pressure of the microreactor. The results shown in Fig. 7B affirm that the GHSV significantly influences the ammonia conversion. The conversion was less than 100% under 400 °C when the GHSV is larger than 35
000 h−1, is determined by the safe operation pressure of the microreactor. The results shown in Fig. 7B affirm that the GHSV significantly influences the ammonia conversion. The conversion was less than 100% under 400 °C when the GHSV is larger than 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. This can be explained by the shortening of the residence time of reactants, because the microchannel shows laminar gas flow and the reaction is controlled by the mass transfer of gas molecules from bulk gas to the catalyst layer. However, on further decreasing the GHSV to less than 35
000 h−1. This can be explained by the shortening of the residence time of reactants, because the microchannel shows laminar gas flow and the reaction is controlled by the mass transfer of gas molecules from bulk gas to the catalyst layer. However, on further decreasing the GHSV to less than 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, no conspicuous improvement in ammonia conversion was observed to offset the decrease in the charging of the total reactants. Therefore, the optimal space velocity for producing the largest capacity is determined as 35
000 h−1, no conspicuous improvement in ammonia conversion was observed to offset the decrease in the charging of the total reactants. Therefore, the optimal space velocity for producing the largest capacity is determined as 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1.
000 h−1.
The effect of ammonia to oxygen volume ratio on the feeding gas reactant is investigated, and the result is shown in Fig. 7C. The GHSV of the gas mixture is fixed as 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, and only the volume ratio of the components is adjusted. The results confirm that ammonia conversion is significantly influenced by the change in the ammonia to oxygen volume ratio. The results show that when the volume concentration of ammonia decreases to 8.56%, ammonia conversion can become 100% under 400 °C. On further decreasing the volume concentration of ammonia to 7.14%, ammonia conversion can become almost 100% under 300 °C. Because the determined NOx selectivity reaches the highest value, which is 88.32%, under 300 °C, and remains constant on further increasing the temperature, the operation value of volume concentration of ammonia is determined as 7.14%, which is identical to the ammonia to oxygen volume ratio of 1
000 h−1, and only the volume ratio of the components is adjusted. The results confirm that ammonia conversion is significantly influenced by the change in the ammonia to oxygen volume ratio. The results show that when the volume concentration of ammonia decreases to 8.56%, ammonia conversion can become 100% under 400 °C. On further decreasing the volume concentration of ammonia to 7.14%, ammonia conversion can become almost 100% under 300 °C. Because the determined NOx selectivity reaches the highest value, which is 88.32%, under 300 °C, and remains constant on further increasing the temperature, the operation value of volume concentration of ammonia is determined as 7.14%, which is identical to the ammonia to oxygen volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.
13.
Nitrogen is an undesired by-product of ammonia oxidation. However, if air is used for ammonia oxidation, the nitrogen in air will greatly hamper the reaction. Therefore, the effect of nitrogen partial pressure in the reactant stream is investigated by adding nitrogen in the feeding gas mixture. The GHSV of the inlet stream during investigation is maintained at 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, and the ammonia flow is fixed at 3 mL min−1. The pure oxygen flow is replaced by a mixture of nitrogen and oxygen as nitrogen–oxygen ratio of 0.10, 0.15, 0.20 and 0.25. The results shown in Fig. 7D confirm the significant influence of the nitrogen partial pressure on ammonia conversion. On increasing the nitrogen percentage from zero to 0.25, the ammonia conversion markedly decreased from 100% to 72% at 400 °C. This sharp decrease in ammonia conversion can be ascribed to the combined action of the partial pressure reduction of reactant oxygen and the reaction inhibition effect of nitrogen as the product. Therefore, it can be concluded that the pure oxygen or oxygen-enriched air is beneficial to ammonia oxidation reaction. In this research, pure oxygen is used as the reactant for the reaction.
000 h−1, and the ammonia flow is fixed at 3 mL min−1. The pure oxygen flow is replaced by a mixture of nitrogen and oxygen as nitrogen–oxygen ratio of 0.10, 0.15, 0.20 and 0.25. The results shown in Fig. 7D confirm the significant influence of the nitrogen partial pressure on ammonia conversion. On increasing the nitrogen percentage from zero to 0.25, the ammonia conversion markedly decreased from 100% to 72% at 400 °C. This sharp decrease in ammonia conversion can be ascribed to the combined action of the partial pressure reduction of reactant oxygen and the reaction inhibition effect of nitrogen as the product. Therefore, it can be concluded that the pure oxygen or oxygen-enriched air is beneficial to ammonia oxidation reaction. In this research, pure oxygen is used as the reactant for the reaction.
Thus, it can be concluded that the optimal operation parameters of the wall-loaded microchannel reactor are as follows: temperature, 300 °C; GHSV, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; catalyst dosage, 85% of the microchannel area; and ammonia to oxygen volume ratio, 1
000 h−1; catalyst dosage, 85% of the microchannel area; and ammonia to oxygen volume ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 without nitrogen. If pure oxygen is replaced with air, the optimal operation temperature should be adjusted to 550 °C.
13 without nitrogen. If pure oxygen is replaced with air, the optimal operation temperature should be adjusted to 550 °C.
3.5. Performance evaluation of wall-loaded microreactor
Here, the NOx selectivity of ammonia oxidation in an optimized microchannel under optimal operation conditions is simulated and measured experimentally. The simulated and experimental ammonia conversion as well as NOx selectivity are plotted in Fig. 8A. It can be observed that the change in the actual ammonia conversion against temperature basically obeys the simulation, but the determined NOx selectivity is completely different. The NOx selectivity reaches the highest value, i.e., 88.32%, under 300 °C, and remains constant, although the reaction temperature continues to increase. Corresponding to this, the simulation displays a continuous increase from 19.73% to 72.05% when the temperature is increased from 250 °C to 400 °C. The maximum value of NOx selectivity is far less than the actual measured value in the microchannel. Such a remarkable and attractive promotion of NOx selectivity is advantageous for the as-prepared microchannel reactor. The reasons behind selectivity promotion are explored and further discussed hereinafter.Pressure drop in the microchannel reactor is determined by both simulation and experiment. Two sets of data can be simultaneously considered. The pressure drop increases sharply from 0.98 to 1.62 kPa on increasing the reaction temperature from 250 to 400 °C. The maximum error at 250 °C between the simulated and experimental values of the pressure drop is as high as 0.31 kPa, whereas at 400 °C, it is only 30 Pa. This error can be ascribed to the pressure drop in the gas inlet and outlet sections on connecting the differential pressure gauge, which is not included in simulation. Under high-operation temperatures, the pressure drop in the microchannel is very high because the inlet and outlet sections are under low temperatures.
Temperature distribution of the catalyst zone of the home-made microreactor is determined and shown in Fig. 8C. Only 4 °C of temperature difference is observed among the different temperature-measuring points. No obvious hot-spot is observed in the entire microreactor. This uniform temperature distribution confirms the excellent thermal conductivity of such Ti-based metal microchannel reactors, which was beneficial and attractive for strongly exothermic reactions.
The stability of the wall-loaded Pt/TiO2/Pt catalyst is also determined by continuously running the microreactor under 350 °C and 270 °C. The selection of 270 °C results from the ignition temperature of ammonia oxidation in microchannel, T50, which is defined as the temperature at which 50% ammonia conversion occurs. The results shown in Fig. 8D affirm that ammonia conversion and NOx selectivity remain constant during 360 h of testing at 400 °C, which confirm its excellent stability. However, ammonia conversion at 270 °C shows only a slight increase from about 50% to about 55% after the first usage, and NOx selectivity increases from 78.43% to about 81%. This favorable increase can be attributed to further activation of Pt species under reaction conditions.
3.6. Improvement in NO selectivity and its mechanism
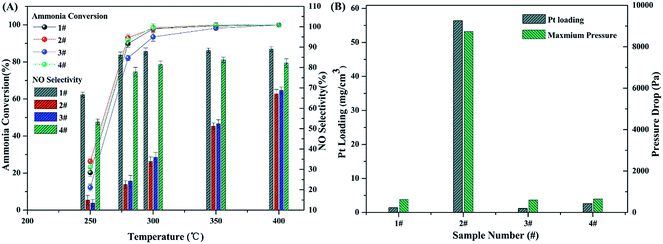
An interesting and attractive result of NOx selectivity improvement in home-made microchannel reactor is observed and worthy of further discussion. Here, a special experiment is designed to explore the mechanism of such improvement. Four different catalyst samples are prepared: wall-loaded Pt/TiO2/Ti on an anodized Ti foil by photodeposition, fine Pt/TiO2 particles, wall-loaded Pt/TiO2/Ti on a calcined Ti foil by photodeposition, and wall-loaded Pt/TiO2/Ti on an anodized Ti foil by impregnation, marked as samples 1#, 2#, 3#, and 4#, respectively. Four indexes—ammonia conversion, NOx selectivity, platinum loading amount, and pressure drop—are selected for performance comparison. The results are shown in Fig. 9.From Fig. 9, it can be confirmed that sample 1#, i.e., the wall-loaded Pt/TiO2/Ti catalyst on the anodized Ti foil by photodeposition, shows the highest NOx selectivity and the least Pt loading and pressure drop among the four samples. However, the difference in the ammonia conversion of the four samples is not significant, as shown in Fig. 9A. Sample 2#, the filled Pt/TiO2 particles in the microchannel, has a slight advantage in terms of ammonia conversion, as shown in Fig. 9B. However, the pressure drop and Pt loading of sample 2# are unacceptably high. Samples 1# and 4# show similar performance, which can be ascribed to the similar structure of the two catalysts. However, the NOx selectivity of sample 1# is slightly higher than that of sample 4#, and Pt loading is slightly less. This small difference between the two samples can be attributed to the difference in the loading approach. The photodeposition process, which can theoretically load a metal as a single-atom layer on the photosensitive support, is more beneficial to immobilize a thinner active species layer than impregnation.
It is easy to understand the low pressure drop of the wall-loaded catalyst without considering the loading approach because of the absence of filling in the microchannel. The similar ammonia conversion of the four samples can be explained by considering that the reaction has reached the equilibrium under operation temperature with adequate catalyst loading. However, the predominant selectivity of sample 1# cannot be explained simply and should be discussed in detail.
An FEM simulation is conducted to investigate the mechanism of increase in NOx selectivity. Wall-loaded Pt/TiO2/Ti catalysts on an anodized Ti foil and a calcined Ti foil are selected for calculation, because although the only difference between the two samples is the nanotube structure, their performances differ greatly. A gas channel with a height of 200 μm and length of 100 μm is used as the simplified 2D model for simulation. Gas enters the model from the left side and leaves from the right side. The difference between the two models, Fig. 10A and B, is the smooth bottom and groove bottom. As shown in Fig. 10A, the bottom is the place for loading active Pt metal and marked in blue, whereas in Fig. 10B, a series of 1 μm × 1 μm blind-hole structure is preset. Only the wall of the blind-hole structure is supposed to load the catalyst, and is marked in blue. Both the models show the same total catalyst loading. In the present work, nitrogen is used as the object for calculation.
Fig. 10 shows the concentration distribution of ammonia and nitrogen in two models. Under the same reaction conditions, it can be observed that the ammonia concentration distribution at the outlet of the two models is almost the same, which confirms the experimental result of similar ammonia conversion. However, the nitrogen concentration at the outlet of the model with a smooth bottom is significantly higher than that obtained with a grooved bottom. This result confirms the high NOx selectivity of the anodized Ti support with a nanotube structure.
Therefore, the mechanism for improving the NOx selectivity of wall-loaded Pt/TiO2/Ti catalysts can be explained by considering the kinetic control of mass transfer, as described in the following section. As shown in Fig. 11A, ammonia oxidation takes place to generate NO when the gas reactants NH3 and O2 enter the microchannel filled with Pt-loaded particles, and the product, NO, can then react with NH3 on the catalyst in the rear to generate by-product N2 under the flowing stream and diffusion, which is the driving force of the reaction. The continuous contact between the reactant and the catalyst active site made it difficult to control the selectivity of the rapid cascade reaction.
For reactions on wall-loaded Pt/TiO2/Ti catalysts on the calcined Ti foil, the entire process is similar. As shown in Fig. 11B, the ammonia oxidation reaction and the ongoing nitrogen production can occur sequentially because the catalyst is exposed under gas flow, although the pressure drop of this structure should be smaller than that in the catalyst-filled fixed-bed reactor. The mass transfer from the bulk gas phase to the catalyst surface is relatively easy. Only the laminar sublayer of gas flow can resist the diffusion of NO molecules. A feasible approach to decrease the amount of undesired by-products is to reduce the loading quantity of the catalyst at the cost of low conversion.
However, because the reaction takes place on wall-loaded Pt/TiO2/Ti on an anodic support, this intractable low selectivity can be alleviated. The blind-hole structure of the nanotubes allows the reactant gas and the product to diffuse between the open top and the main gas stream. As shown in Fig. 11C, the gas reactant swept from the open top of the nanotubes diffuses into the tubes and reacts at the Pt active sites located inside the tubes. The concentration of the product NO increases inside the nanotubes, forming a concentration gradient that diffuses the NO molecules out of the nanotubes and block their re-entering. Thus, the actual residence time of NO is efficiently shortened, and the amount of the by-product is significantly reduced. The difference in the loading method, impregnation or photodeposition, changes only the loading amount of Pt, and consequently slightly changes the selectivity.
4. Conclusion
In this research, a home-made microchannel reactor was designed based on a wall-loaded catalyst by the photodeposition of metal active species on an anodic Ti foil. Ammonia oxidation reaction was used as the probe reaction to evaluate the performance of such a microchannel reactor and to investigate the advantages of the wall-loaded Pt/TiO2/Ti catalyst. A porous TiO2 nanotube array was constructed on the surface by the anodization of the Ti foil and was used for loading Pt by photoreduction. The as-prepared wall-loaded catalyst was assembled into the microreactor as a side wall of the microchannel and by catalyzing the ammonia oxidation. Simulation and experimental results showed that the complete conversion of ammonia can be realized under a relatively moderate temperature of 300 °C, GHSV of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, catalyst loading percent of 85% of the total channel area, and ammonia to oxygen volume ratio of 1
000 h−1, catalyst loading percent of 85% of the total channel area, and ammonia to oxygen volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 without nitrogen in the microchannel of length 80 mm, width 1.0 mm, and height 0.3 mm. Flow resistance in the microchannel was significantly small and the temperature distribution was uniform. It was worth noting that NOx selectivity in such microchannel reactors with wall-loaded catalysts remarkably improved to 88.32% under 300 °C. Such a significant improvement in selectivity can be ascribed to the unique blind-hole structure of the TiO2 nanotube, which effectively shortened any further contact of product NO with the catalyst, thereby blocking the successive reaction to generate by-product N2. This selectivity-improving effect of the anodic nanotube support is attractive and valuable for application in rapid gas to solid exothermic reactions. The stability of such a self-designed reactor with a wall-loaded Pt/TiO2/Ti catalyst was also investigated; no remarkable performance decay was observed even after 360 h of testing. Because of these advantages, the use of a microchannel reactor with a wall-loaded catalyst on anodic metal presents an attractive prospect for large-scale applications in the field of rapid gas to solid exothermic reactions.
13 without nitrogen in the microchannel of length 80 mm, width 1.0 mm, and height 0.3 mm. Flow resistance in the microchannel was significantly small and the temperature distribution was uniform. It was worth noting that NOx selectivity in such microchannel reactors with wall-loaded catalysts remarkably improved to 88.32% under 300 °C. Such a significant improvement in selectivity can be ascribed to the unique blind-hole structure of the TiO2 nanotube, which effectively shortened any further contact of product NO with the catalyst, thereby blocking the successive reaction to generate by-product N2. This selectivity-improving effect of the anodic nanotube support is attractive and valuable for application in rapid gas to solid exothermic reactions. The stability of such a self-designed reactor with a wall-loaded Pt/TiO2/Ti catalyst was also investigated; no remarkable performance decay was observed even after 360 h of testing. Because of these advantages, the use of a microchannel reactor with a wall-loaded catalyst on anodic metal presents an attractive prospect for large-scale applications in the field of rapid gas to solid exothermic reactions.
Acknowledgements
We appreciated the financial support from the National Natural Science Foundation of China (21176157).References
- W. Ehrfeld, V. Hessel and V. Haverkamp, Microreactors, Wiley Online Library, 2000 Search PubMed.
- Y. Wang and J. D. Holladay, Microreactor technology and process intensification, Amer Chemical Society, 2005 Search PubMed.
- L. Kiwi-Minsker and A. Renken, Catal. Today, 2005, 110, 2–14 CrossRef CAS.
- A. Pohar and I. Plazl, Chem. Biochem. Eng. Q., 2009, 23, 537–544 CAS.
- K. Geyer, T. Gustafsson and P. H. Seeberger, Synlett, 2009, 2382–2391 CAS.
- C. G. Frost and L. Mutton, Green Chem., 2010, 12, 1687–1703 RSC.
- M. Lamblin, L. Nassar-Hardy, J. C. Hierso, E. Fouquet and F. X. Felpin, Adv. Synth. Catal., 2010, 352, 33–79 CrossRef CAS.
- V. Meille, Appl. Catal., A, 2006, 315, 1–17 CrossRef CAS.
- A. Cybulski and J. A. Moulijn, Structured catalysts and reactors, CRC Press, 2005 Search PubMed.
- P. Avila, M. Montes and E. E. Miro, Chem. Eng. J., 2005, 109, 11–36 CrossRef CAS.
- V. Hessel, P. Angeli, A. Gavriilidis and H. Löwe, Ind. Eng. Chem. Res., 2005, 44, 9750–9769 CrossRef CAS.
- G. Wieβmeier and D. Hönicke, Ind. Eng. Chem. Res., 1996, 35, 4412–4416 CrossRef.
- J. Ganley, K. Riechmann, E. Seebauer and R. Masel, J. Catal., 2004, 227, 26–32 CrossRef CAS.
- J. C. Ganley, E. Seebauer and R. I. Masel, AIChE J., 2004, 50, 829–834 CrossRef CAS.
- J. Hereijgers, G. Desmet, T. Breugelmans and W. De Malsche, Microelectron. Eng., 2015, 132, 1–13 CrossRef CAS.
- Z. Ni, E. Seebauer and R. I. Masel, Ind. Eng. Chem. Res., 2005, 44, 4267–4271 CrossRef CAS.
- D. Regonini, C. Bowen, A. Jaroenworaluck and R. Stevens, Mater. Sci. Eng., R, 2013, 74, 377–406 CrossRef.
- V. Galstyan, E. Comini, G. Faglia and G. Sberveglieri, Sensors, 2013, 13, 14813–14838 CrossRef PubMed.
- B. L. Ellis, P. Knauth and T. Djenizian, Adv. Mater., 2014, 26, 3368–3397 CrossRef CAS PubMed.
- S. Minagar, C. C. Berndt, J. Wang, E. Ivanova and C. Wen, Acta Biomater., 2012, 8, 2875–2888 CrossRef CAS PubMed.
- P. Roy, S. Berger and P. Schmuki, Angew. Chem., Int. Ed., 2011, 50, 2904–2939 CrossRef CAS PubMed.
- M. Yada and Y. Inoue, in Inorganic and Metallic Nanotubular Materials, Springer, 2010, pp. 117–133 Search PubMed.
- D. P. Singh and N. Ali, Sci. Adv. Mater., 2010, 2, 295–335 CrossRef CAS.
- W. Jiang, J. He, J. Zhong, J. Lu, S. Yuan and B. Liang, Appl. Surf. Sci., 2014, 307, 407–413 CrossRef CAS.
- F. Möllers, H. Tolle and R. Memming, J. Electrochem. Soc., 1974, 121, 1160–1167 CrossRef.
- K. Tanaka, K. Harada and S. Murata, Sol. Energy, 1986, 36, 159–161 CrossRef CAS.
- S. Sakthivel, M. Shankar, M. Palanichamy, B. Arabindoo, D. Bahnemann and V. Murugesan, Water Res., 2004, 38, 3001–3008 CrossRef CAS PubMed.
- J.-M. Herrmann, J. Disdier and P. Pichat, J. Catal., 1988, 113, 72–81 CrossRef CAS.
- Q.-H. Zhang and L. Gao, Acta Chim. Sin., 2005, 63, 65–70 CAS.
- D. Li, J. T. McCann, M. Gratt and Y. Xia, Chem. Phys. Lett., 2004, 394, 387–391 CrossRef CAS.
- S. C. Chan and M. A. Barteau, Langmuir, 2005, 21, 5588–5595 CrossRef CAS PubMed.
- R. Zhao, R. Ding, S. Yuan, W. Jiang and B. Liang, Int. J. Hydrogen Energy, 2011, 36, 1066–1073 CrossRef CAS.
- R. Srinivasan, I. Hsing, P. E. Berger, K. F. Jensen, S. L. Firebaugh, M. A. Schmidt, M. P. Harold, J. J. Lerou and J. F. Ryley, AIChE J., 1997, 43, 3059–3069 CrossRef CAS.
- R. B. Bird, W. E. Stewart and E. N. Lightfoot, Transport phenomena, JohnWiley & Sons, New York, 2002 Search PubMed.
- T. Pignet and L. Schmidt, J. Catal., 1975, 40, 212–225 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |