Carbonyl iron coated with a sulfobetaine moiety as a biocompatible system and the magnetorheological performance of its silicone oil suspensions
Miroslav Mrlík*a,
Markéta Ilčíkovábc,
Martin Cveka,
Vladimír Pavlíneka,
Anna Zahoranováb,
Zuzana Kronekováb and
Peter Kasak*c
aCentre of Polymer Systems, University Institute, Tomas Bata University in Zlín, Třída T. Bati 5678, 760 01 Zlín, Czech Republic. E-mail: mrlik@cps.utb.cz
bPolymer Institute, Slovak Academy of Sciences, Dúbravska cesta 9, 845 45, Bratislava 45, Slovakia
cCentre for Advanced Materials, Qatar University, P. O. BOX 2713, Doha, Qatar. E-mail: peter.kasak@qu.edu.qa
First published on 24th March 2016
Abstract
In this study, surface modification of carbonyl iron (CI) particles with sulfobetaine moieties (SBE) was performed by the silanization of activated CI to form stable CI–SBE particles. The modification led to a significant improvement of the thermo-oxidation stability and a negligible suppression of the magnetization of the particles, as revealed by thermogravimetric analysis and vibrating sample magnetometry, respectively. The effect of a magnetic field and temperature on the magnetorheological performance of particle suspensions was investigated using a rotational rheometer in order to clarify the suitability of these systems for the local embolization of blood veins. The suspension based on CI–SBE exhibited a pseudoplastic behaviour and a tunable yield stress in a range from 0.3–4 kPa at the normal human body temperature. Moreover, cell viability for fibroblasts and macrophages was examined via MTT assay, which revealed their suitability for the intended applications for the local embolization of blood veins.
1. Introduction
Magnetorheological (MR) suspensions belong to smart responsive materials, in which the application of an external magnetic field permits reversible transitions between liquid-like and solid-like states.1–3 These two-phase systems usually consist of ferromagnetic micro-sized particles4–8 in a liquid carrier. The MR suspensions exhibit Newtonian-like behaviour in the absence of an external magnetic field while in the presence of magnetic field, form-oriented, internal, chain-like structures due to induced magnetic dipoles along the streamlines of the applied magnetic field. The formation of the internal structures results in flow constraint, and suspensions behave pseudoplasticly, exhibiting yield stress, increased viscosity and viscoelastic moduli by several orders of magnitude.9–14 The rapid response to the external field, high MR performance, high chemical and thermo-oxidation stability, slow sedimentation and facile re-dispersibility of the magnetic particles are desired properties of MR fluids. These requirements can be met by various approaches, such as the preparation of core–shell particles, inorganic–inorganic15,16 or inorganic–organic, where low molecular weight substances are used,17 either random polymer coatings18,19 or controllable polymer coatings.20–22In the last decade, MR suspensions have been recognized as promising systems for applications in automobiles, such as brakes, dampers, clutches and shock absorbers or torque transducers.23–25 However, another utilization of these systems was found in connection with medical science, i.e., artificial muscles or the local embolization of blood veins.26–28 In the last mentioned application, the MR fluid is injected into a blood vessel leading to a tumour, followed by the application of a magnetic field over the tumour area. The magnetic particles form a solid seal that blocks the tumour from its blood supply, leading to tumour necrosis.28 In this respect, the biocompatibility and low cytotoxicity of MR suspension components are desirable. The coatings with silver citrate or starch derivatives have been used to ensure biocompatibility and reduce electrostatic repulsive interactions with red blood cells while retaining a sufficient MR response.28,29 Suppressed cytotoxicity was also observed in CI with controllably-grafted poly(glycidylmethacrylate) brushes.30 Moreover, the “grafting from” approach enables control of the polymer shell thickness, and thus can effectively tune the sedimentation, redispersibility and stability of the magnetic particles.21 However, the polymer coatings reduce magnetization of the particles.31,32 Therefore, in this study a self-assembled monolayer coating was introduced onto the CI surface. The coating consists of sulfobetaine moieties, which are known to exhibit non-fouling properties on proteins, platelets adhesion resistance, and hemocompatibility,33,34 and they promote the intracellular uptake of the (nano)particles.35,36 These properties arise mainly from the internally-balanced charge of coating moieties and the strong electrostatic interaction with water, which form a highly hydrophilic surface, preventing interactions with charged bio molecules.37
Sulfobetaine coatings have been used mainly for designing various biocompatible, non-fouling functional materials.35,36,38,39 Moreover, other advanced applications such as a smart electro-responsive material, have been explored.40
In this study, the CI particle surface was modified with an outer sulfobetaine shell in order to improve thermo-oxidation stability and biocompatibility while retaining the high magnetic saturation of the CI particles. A sulfobetaine-based, self-assembly monolayer was formed by silanization, and the successful modification was confirmed via Fourier transform infrared spectroscopy (FTIR) and energy dispersive spectroscopy (EDS). The magnetic properties were investigated by vibration sample magnetometry (VSM), and no significant effect on magnetization was observed after the modification. The thermo-oxidation stability of such particles was measured using thermogravimetric analysis (TGA), and cytotoxicity was evaluated via the MMT assay method. The MR performance of the suspensions was measured at various temperatures and studied. We believe that this modification approach makes modified CI particles promising candidates for smart biomedical applications such as the local embolization of blood veins.
2. Experimental
2.1 Materials
3-(4,5-Dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Calbiochem (Merck Millipore, Darmstadt, Germany). Dulbecco's Modified Eagle Medium (DMEM), RPMI-1640, fetal bovine serum (FBS), horse serum (HS), streptomycin, penicillin, and L-glutamine were purchased from Gibco (Life Technologies, Grand Island, NY, USA). Mouse 3T3 fibroblasts and macrophage P388.D1 were both from DSMZ, Braunschweig, Germany. Diethyl ether, [3-(methacrylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide inert salt (SBE), azobisisobutyronitrile (AIBN), (3-mercaptopropyl)trimethoxysilane (MTMS), acetone and dimethyl sulfoxide (DMSO) were purchased from Aldrich and used as received. Hydrochloric acid (HCl, 35%, p.a.), ethanol and methanol were obtained from Penta Labs (Czech Republic). The CI powder (HQ grade) was purchased from BASF Corporation (Germany). Silicone oil used for the preparation of the MR suspensions was Lukosiol M15 (Chemical Works Kolín, Czech Republic; dynamic viscosity ηc = 14.1 mPa s, density dc = 0.970 g cm−3, relative permittivity ε′ = 2.89, loss factor tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 0.0001).
δ = 0.0001).
2.2 Modification of carbonyl iron particles
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), then MTMS (1 mL, 5.5 mmol) and a catalytic amount of AIBN dissolved in 1 mL ethanol was added. The reaction mixture was stirred and irradiated at 365 nm (UV lamp) for 2 hours at an ambient temperature under an argon atmosphere. The reaction mixture was precipitated into a dried diethyl ether. The precipitate was separated via centrifugation (5000 rpm, 5 min), and the decanted solvent was discharged. The precipitation into the dried diethyl ether was repeated, and a white powder product was obtained after drying in a vacuum at an ambient temperature for 2 hours.
1), then MTMS (1 mL, 5.5 mmol) and a catalytic amount of AIBN dissolved in 1 mL ethanol was added. The reaction mixture was stirred and irradiated at 365 nm (UV lamp) for 2 hours at an ambient temperature under an argon atmosphere. The reaction mixture was precipitated into a dried diethyl ether. The precipitate was separated via centrifugation (5000 rpm, 5 min), and the decanted solvent was discharged. The precipitation into the dried diethyl ether was repeated, and a white powder product was obtained after drying in a vacuum at an ambient temperature for 2 hours.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 1 g SBE–Si. The reaction mixture was stirred and refluxed for 6 hours. Then the particles were washed with 100 mL ethanol three times and dried at an ambient temperature overnight to obtain app. 11 g of CI–SBE product, as seen in Scheme 2.
1) and 1 g SBE–Si. The reaction mixture was stirred and refluxed for 6 hours. Then the particles were washed with 100 mL ethanol three times and dried at an ambient temperature overnight to obtain app. 11 g of CI–SBE product, as seen in Scheme 2.2.3 Characterization
The structure of the SBE–Si was confirmed by 1H NMR using a 400 MHz VNMRS Varian NMR spectrometer (Varian Inc. since 1999 part of Agilent, Japan) equipped with a 5 mm1H–19F/15N–31P PFG AutoX DB NB probe at 25 °C. Deuterated methanol was used as a solvent and the concentration of the sample was approximately 20 mg in 0.7 mL solvent.FTIR spectra were obtained from a Nicolet FTIR spectrometer (Nicolet 6700, USA) equipped with an ATR accessory. The measurement was performed at laboratory temperature using a germanium crystal in the region of 4000–500 cm−1.
The morphology and structure were observed by the scanning electron microscope (SEM) Vega II/LMU (Tescan, Czech Republic) operated at 10 kV equipped with an EDS spectroscope. The magnetic properties were studied using a vibrating sample magnetometer VSM 7400 (Lake Shore, United States).
Thermo-oxidative stability, an important characteristic of the MR particles, was investigated with the help of a TGA (TA Instruments Q500, USA) under an air atmosphere at a heating rate of 10 K min−1.
2.4 Suspension preparation
Bare CI and the CI–SBE particles were dispersed in silicone oil Lukosiol M 15 at a fixed particle concentration of 60 wt%. The concentration was chosen to examine and compare the MR performance with the literature data. Lukosiol M 15 was chosen since the value of its viscosity is close to blood viscosity, which was found to be 4 mPa s at 37 °C. In each case, the suspensions were mechanically stirred and then sonicated for 30 s before each measurement.2.5 Rheological properties
The rheological properties in the steady state flow were measured under an external magnetic field in the range of 0–300 mT using a rotational rheometer Physica MCR 502 (Anton Paar GmbH, Austria) equipped with a Physica MRD 170/1T magneto-cell. The true magnetic flux density was measured using a Hall probe, and the temperature was controlled by an external thermostat (Julabo, Germany). All rheological experiments were performed at temperatures of 25 and 36.8 °C.2.6 Cytotoxicity investigation
3. Results and discussion
3.1 Synthesis and characterization of CI–SBE
The sulfobetaine-based coating was employed in this study to enhance the sedimentation stability and to ensure the biocompatibility of the CI particles. As depicted in Scheme 2, the modification of the CI surface was performed by silanization with the sulfobetaine derivative SBE–Si to form CI–SBE.The SBE–Si was synthesized via a thiol-ene click reaction as can be seen in Scheme 1, i.e., the radical mediated addition of a thiol to a vinyl group.42 The SBE–Si was employed for the silanization of activated CI particles, since the silanization is a simple method frequently used for the preparation of core–shell systems.43
In Fig. 1, the FTIR spectra of bare CI, neat SBE–Si and modified CI–SBE particles are compared. The novel absorption bands corresponding to C–H bonds are visible at around 3000 cm−1. New absorption bands appeared also at 1267 cm−1 and 1118 cm−1, which correspond to sulphate and ammonium groups, respectively. The absorption of the C–S bond for SBE–Si and SBE–CI is observed at 720 and 736 cm−1 respectively, and the peaks are slightly shifted in the SBE–CI to higher wavenumbers due to the presence of the CI particles.13
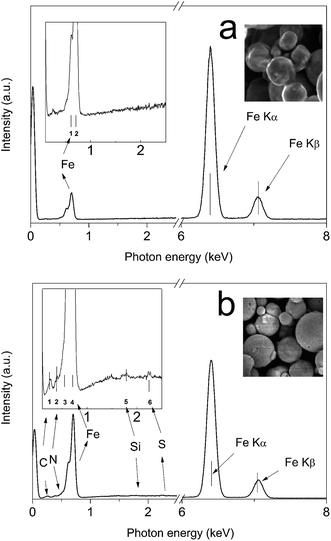
EDS spectroscopy was employed to investigate the presence of elements on the particle surface. In Fig. 2, the EDS spectra of bare CI particles and modified CI–SBE particles are shown. Nearly pure CI with characteristic emission peaks of iron at 0.705 keV representing Lα and 6.398 keV and 7.05 keV representing Kα and Kβ, respectively can be clearly seen. The analysis of the CI–SBE (Fig. 2b) revealed the additional presence of carbon at 0.277 keV, nitrogen at 0.392, silicone at 1.739 keV, and finally sulphur at 2.307 keV, all of them representing Kα emissions and originating from the SBE–Si modification.
VSM measurements were performed in order to investigate the effect of the modification on the magnetic properties of the CI particles. In Fig. 3, the magnetization curves of bare CI and CI–SBE are shown. The magnetization obtained for the magnetic field strength 9566 Oe reached 188 emu g−1 and 183 emu g−1, for bare CI and modified CI–SBE, respectively. The slight decrease, 2.7%, was expected since the modification forms a non-magnetic shell by a self-assembled monolayer. However, such a decrease in magnetization of the particles is negligible and has no negative impact on the MR response. A similar trend after the formation of a shell on the particles was reported by Mrlik et al.17,44
 | ||
Fig. 3 Magnetization curves of bare CI particles (solid line) and CI–SBE (dotted line). The inset graph corresponds to magnification in the magnetic field range of 7000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. 000 Oe. | ||
3.2 Thermo-oxidation stability
In Fig. 4, the thermo-oxidation stability of bare CI and modified CI–SBE particles is plotted. Both particle types exhibited an increasing trend in sample mass due to iron oxide formation in a temperature range from 250 °C to 700 °C. The sharp gain in mass of bare CI starts at 250 °C and can be assigned to the formation of different iron oxide forms, i.e., FeO and Fe3O4. The mass of bare CI increased by 38% at 800 °C, which corresponds to the formation of Fe3O4.45 On the other hand, the modified CI–SBE exhibited a slower mass increase with a total gain of 20% at 800 °C. Apparently, the self-assembly monolayer prevents CI oxidation, resulting in the formation of a less-oxidized form, i.e., FeO. | ||
| Fig. 4 TGA curves of bare CI particles (solid line) and modified CI–SBE particles (dashed line) measured in air atmosphere. | ||
3.3 MR properties
VSM studies revealed sufficient post-modification particle magnetization and the capability of this material to be applied as a component of MR suspensions. MR suspensions are promising candidates for applications in medicine. Therefore, the effect of the external magnetic field on the rheological characteristics of the MR suspensions, including yield stress, rate of chain formation and their redispersibility, are crucial parameters for potential applications.46,47In Fig. 5a and b, the dependences of the shear stress, τ, on the shear rate, ![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) , of suspensions containing bare CI particles as well as CI–SBE particles measured at 25 °C are shown. Both suspensions exhibit pseudoplastic behavior in the absence of the external magnetic field in the investigated shear rate range. This is important, since the suspensions are planned to be injected into the human body, and flow characteristics indicate that high shear rates usually applied during the injection significantly decrease the suspension viscosity, making this procedure possible. Upon the application of the external magnetic field, the shear stress increases significantly, and the performance of the MR changes from Newtonian to Bingham as a consequence of the formation of internal structures. The solidification of the internal chain-like structures can be further enhanced by increasing the intensity of the external magnetic field, expressed as magnetic flux density (B). Both suspensions exhibited the same trend and reached comparable values of shear stresses at all investigated B.
, of suspensions containing bare CI particles as well as CI–SBE particles measured at 25 °C are shown. Both suspensions exhibit pseudoplastic behavior in the absence of the external magnetic field in the investigated shear rate range. This is important, since the suspensions are planned to be injected into the human body, and flow characteristics indicate that high shear rates usually applied during the injection significantly decrease the suspension viscosity, making this procedure possible. Upon the application of the external magnetic field, the shear stress increases significantly, and the performance of the MR changes from Newtonian to Bingham as a consequence of the formation of internal structures. The solidification of the internal chain-like structures can be further enhanced by increasing the intensity of the external magnetic field, expressed as magnetic flux density (B). Both suspensions exhibited the same trend and reached comparable values of shear stresses at all investigated B.
Fig. 5c and d represent the MR behaviour of the suspensions at the average human body temperature (36.8 °C) due to the investigation of the rheological behaviour at a temperature relevant for medical applications. In the absence of the external magnetic field, both suspensions exhibited pronounced pseudoplastic behaviour, which is desirable from the point of view of an injectable MR material. Upon the application of a magnetic field, internal structures developed, but the shear stresses reached lower values. At an elevated temperature, the Brownian thermal motion affected the overall MR performance by disturbing the formation of the internal structures in the suspensions. Nevertheless, the values of shear stresses are still high enough for potential medical applications, since Sheng et al.48 reported the yield stresses of 40 mm Hg, i.e., 5.3 kPa, and 200 mm Hg, i.e., 27 kPa, are sufficient for the blockage of blood capillaries and main arteries, respectively.
Fig. 6a and b show a deeper investigation of the mechanism for internal chain-like structure formation for the MR suspensions. The yield stress, τy, as a measure of the rigidity of the internal structures, is significantly dependent on the magnetic flux density, B, especially in case of lower values of B. The data fitted with the power law model, τy ∝ Ba, enabled clarification of the mechanism of the structure formation through assessment of the parameter a.22,26,36 There are two mechanisms: the dipole and local saturation mechanism.49,50 In the case of the dipole mechanism, the particles are effectively involved in the formation of the chain-like structures, and τy varies with B2. This phenomenon can be observed at a rather low B. When τy varies with B1.5, some particles do not participate in the chain-like structure and form clusters with saturated magnetization. The local saturation is observed at a higher B and appears once the critical magnetic flux density, Bc, is exceeded. The parameters a of the power-law model fits are summarized in Table 1.
| Temperature | 25 [°C] | 36.8 [°C] | ||
| Sample code | CI | CI–SBE | CI | CI–SBE |
| Low magnetic flux densities | 2.07 | 2.19 | 2.14 | 2.21 |
| High magnetic flux densities | 1.45 | 1.22 | 1.42 | 1.20 |
Table 1 and Fig. 6 show that CI and the CI–SBE exhibited dipole structure formation and a is equal to 2.07 and 2.19 at room temperature at low B, and local saturation with a equals to 1.45 and 1.22 at high B. The value for the CI–SBE based suspension, 1.22, is more deviated from theory, indicating larger imperfections in the chain-like internal structure formation. When compared to bare CI, the CI–SBE exhibited lower τy and higher Bc that point to suppressed local saturation due to the self-assembly monolayer shell preventing particle aggregation.51
A similar trend was observed at an elevated temperature (Fig. 6b), however the deviations from the model values of a are more pronounced. This indicates a significantly increased number of imperfections in the internal structures formed upon application of the magnetic field, which results in decreased values of the τy when compared to the τy obtained at room temperature.
3.4 Cytotoxicity of bare CI and CI–SBE particles
Since the MR investigation of the CI–SBE showed sufficient MR performance, this material can be utilized as a system for the local embolization of blood veins.26–28 This material will be in contact with living cells, and thus an investigation of the cytotoxicity of particles after modification is required. As previously determined by other authors,20,52,53 iron nanoparticles as well as bare CI particles and their extracts were found to be non-toxic. In this study, the cytotoxicity of both particles was tested in the range of concentration from 1 μg mL−1 up to 1000 μg mL−1 using 3T3 murine fibroblasts and macrophages (P388.D1). Cell viability after 24 hours of incubation is plotted in Fig. 7. In case of concentrations ranging from 1 to 500 μg mL−1, the cell viability was above 80% for the CI as well as CI–SBE, which is assigned as no cytotoxicity effect. The cytotoxicity of the particles was also tested on macrophages, and the cell viability after 24 hours of incubation is depicted in Fig. 8. Macrophages represent the cells of the first line in immune defense and are known to phagocyte the nanoparticles, eliminating them from the blood stream.54,55 Concentrations up to 100 μg mL−1 represent safe values in regard to the cell viability. The decreased cell viability of macrophages at higher concentrations in the case of the CI–SBE could be associated with the proper dispergation of the individual CI–SBE particles, while bare CI particles form aggregates too large to be phagocytized.56 The individual CI–SBE particles are better recognized by macrophages and phagocytized, resulting in a macrophage overload and possibly leading to the loss of macrophage membrane plasticity and a phenomenon known as incomplete phagocytosis, which subsequently leads to cell death.57 | ||
| Fig. 7 Cell viability (fibroblasts 3T3) at various concentrations of the bare CI and the CI–SBE particles. | ||
 | ||
| Fig. 8 Cell viability (macrophages P388.D1) at various concentrations of the bare CI and the CI–SBE particles. | ||
Therefore, the CI–SBE particles provide a system with no cytotoxicity to fibroblasts and macrophages up to 500 μg mL−1 and 100 μg mL−1, respectively, making them promising materials for the local embolization of blood veins.
4. Conclusion
Modification of the CI particle surface with a sulfobetaine-based, self-assembly monolayer via silanization to form CI–SBE was performed. The CI–SBE exhibited more than a 100 °C enhancement in thermo-oxidation stability. The negligible effect of the modification on the magnetization properties indicates that such particles are promising for their application in magnetorheology. A magnetorheological investigation at human body temperature proved that the MR performance is sufficient for the local embolization of blood veins with a tunable yield stress from 0.3–4 kPa at a magnetic flux density from 50–300 mT. Cytotoxicity measurements proved negligible up to concentrations of 500 μg mL−1 and 100 μg mL−1, to fibroblasts and macrophages, respectively.Acknowledgements
This work was made possible by NPRP grant no. NPRP-6-381-1-078 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors. Furthermore, this work was supported by the Ministry of Education, Youth and Sports of the Czech Republic – Program NPU I (LO1504). Author M. C. further thanks the Internal Grant Agency of the Czech Republic (project No. IGA/CPS/2016/008) for financial support. Author Z. K. also gratefully acknowledges the Slovak Grant Agency, VEGA, (project No. 2/0156/15).References
- G. Bossis, S. Lacis, A. Meunier and O. Volkova, J. Magn. Magn. Mater., 2002, 252, 224 CrossRef CAS.
- D. J. Klingenberg, J. C. Ulicny and M. A. Golden, J. Rheol., 2007, 51, 883 CrossRef CAS.
- A. J. F. Bombard, M. Knobel and M. R. Alcantara, Int. J. Mod. Phys. B, 2007, 21, 4858 CrossRef CAS.
- B. J. Park, F. F. Fang and H. J. Choi, Soft Matter, 2010, 6, 5246 RSC.
- J. de Vicente, D. J. Klingenberg and R. Hidalgo-Alvarez, Soft Matter, 2011, 7, 370 RSC.
- W. L. Zhang and H. J. Choi, J. Appl. Phys., 2014, 115, 17B508 CrossRef.
- Y. D. Liu and H. J. Choi, J. Appl. Phys., 2014, 115, 17B529 CrossRef.
- G. R. Iglesias, M. T. Lopez-Lopez, A. V. Delgado and J. D. G. Duran, Rev. Sci. Instrum., 2011, 82, 073906 CrossRef CAS PubMed.
- A. J. F. Bombard and J. V. R. Teodoro, Int. J. Mod. Phys. B, 2011, 25, 943 CrossRef CAS.
- M. Sedlacik, V. Pavlinek, P. Saha, P. Svrcinova and P. Filip, Mod. Phys. Lett. B, 2012, 26, 1150013 CrossRef.
- J. de Vicente and J. Ramirez, J. Colloid Interface Sci., 2007, 316, 867 CrossRef CAS PubMed.
- J. L. Viota, F. Gonzales-Caballero, J. D. G. Duran and A. V. Delgado, J. Colloid Interface Sci., 2007, 309, 135 CrossRef CAS PubMed.
- M. Mrlik, M. Sedlacik, V. Pavlinek, P. Bazant, P. Saha, P. Svrcinova and P. Filip, J. Appl. Polym. Sci., 2013, 128, 2977 CrossRef CAS.
- K. von Pfeil, M. D. Graham, D. J. Klingenberg and J. F. Morris, Phys. Rev. Lett., 2002, 88, 188301 CrossRef PubMed.
- M. Machovsky, M. Mrlik, I. Kuritka, V. Pavlinek and V. Babayan, RSC Adv., 2014, 4, 996 RSC.
- F. F. Fang, Y. D. Liu, H. J. Choi and Y. Seo, ACS Appl. Mater. Interfaces, 2011, 3, 3487 CAS.
- M. Mrlik, M. Ilcikova, V. Pavlinek, J. Mosnacek, P. Peer and P. Filip, Colloid Polym. Sci., 2014, 292, 2137–2143 CAS.
- M. Sedlacik, V. Pavlinek, P. Saha, P. Svrcinova, P. Filip and J. Stejskal, Smart Mater. Struct., 2010, 19, 115008 CrossRef.
- J. H. Park, B. D. Chin and O. O. Park, J. Colloid Interface Sci., 2001, 240, 349 CrossRef CAS PubMed.
- M. Cvek, M. Mrlik, M. Ilcikova, T. Plachy, M. Sedlacik, J. Mosnacek and V. Pavlinek, J. Mater. Chem. C, 2015, 3, 4646–4656 RSC.
- B. Hu, A. Fuchs, S. Huseyin, F. Gordaninejad and C. Evrensel, Polymer, 2006, 47, 7653–7663 CrossRef CAS.
- B. J. Park, M. K. Hong and H. J. Choi, Colloid Polym. Sci., 2009, 287, 501–504 CAS.
- D. Case, B. Taheri and E. Richer, IEEE ASME Trans. Mechatron., 2013, 18, 96 CrossRef.
- B. Gonenc and H. Gurocak, Mechatronics, 2012, 22, 1161 CrossRef.
- D. M. Wang, Y. F. Hou and Z. Z. Tian, Smart Mater. Struct., 2013, 22, 025019 CrossRef.
- E. Kostamo, M. Focchi, E. Guglielmino, J. Kostamo, C. Semini, J. Buchli, M. Pietola and D. Caldwell, J. Mech. Des., 2014, 136, 021003 CrossRef.
- M. Sedlacik, R. Moucka, Z. Kozakova, N. E. Kazantseva, V. Pavlinek, I. Kuritka, O. Kaman and P. Peer, J. Magn. Magn. Mater., 2013, 326, 7 CrossRef CAS.
- G. A. Flores and J. Liu, J. Intell. Mater. Syst. Struct., 2002, 13, 640–646 CrossRef.
- J. Liu, G. A. Flores and R. Sheng, J. Magn. Magn. Mater., 2001, 225, 209–217 CrossRef CAS.
- M. Cvek, M. Mrlik, M. Ilcikova, J. Mosnacek, V. Babayan, Z. Kuceková, P. Humpolicek and V. Pavlinek, RSC Adv., 2015, 5, 72816–72824 RSC.
- J. L. Arias, M. Lopez-Viota, M. A. Ruiz, J. Lopez-Viota and A. V. Delgado, Int. J. Pharm., 2007, 339, 237–245 CrossRef CAS PubMed.
- S. Y. Kim, S. H. Kwon, Y. D. Liu, J. S. Lee, C. Y. You and H. J. Choi, J. Mater. Sci., 2014, 49, 1345–1352 CrossRef CAS.
- J. Huang and W. Xu, Appl. Surf. Sci., 2010, 256, 3921–3927 CrossRef CAS.
- Y. Jiang, Q. F. Hou, B. L. Liu, S. Jiang and S. C. Lin, Colloids Surf., B, 2004, 36, 19–26 CrossRef CAS PubMed.
- A. Lashevsky, Polymers, 2014, 6, 1544 CrossRef.
- L. Mi and S. Jiang, Angew. Chem., Int. Ed., 2014, 53, 1746–1750 CrossRef CAS PubMed.
- Q. Shao and S. Y. Jiang, J. Phys. Chem. B, 2014, 118, 7630–7637 CrossRef CAS PubMed.
- P. Jolly, N. Formisano, J. Tkáč, P. Kasák, C. G. Frost and P. Estrela, Sens. Actuators, B, 2015, 209, 306–312 CrossRef CAS.
- T. Bertok, L. Klukova, A. Sediva, P. Kasak, V. Semak, M. Micusik, M. Omastova, L. Chovanová, M. Vlček, R. Imrich, A. Vikartovska and J. Tkáč, Anal. Chem., 2013, 85, 7324–7332 CrossRef CAS PubMed.
- M. Ilcikova, M. Mrlik, V. Babayan and P. Kasak, Graphene Oxide Modified by Betaine Moieties for Improvement of Electrorheological Performance, RSC Adv., 2015, 5, 57820–57827 RSC.
- Y. Tonomura, T. Kubota and T. Honma, US pat., US20120203020 A1, 2012.
- W. X. Xi, T. F. Scott, C. J. Klaxin and C. N. Bowman, Adv. Funct. Mater., 2014, 24, 2572–2590 CrossRef CAS.
- M. B. Gawande, A. Goswami, T. Asefa, H. Z. Guo, A. V. Biradar, D. L. Peng, R. Zboril and R. S. Varma, Chem. Soc. Rev., 2015, 44, 7540–7590 RSC.
- M. Mrlik, M. Ilcikova, V. Pavlinek, J. Mosnacek, P. Peer and P. Filip, J. Colloid Interface Sci., 2013, 396, 146–151 CrossRef CAS PubMed.
- M. A. Abshinova, N. E. Kazantseva, P. Saha, I. Sapurina, J. Kovarova and J. Stejskal, Polym. Degrad. Stab., 2008, 93, 1826–1831 CrossRef CAS.
- Y. D. Liu, F. F. Fang and H. J. Choi, Colloid Polym. Sci., 2011, 289, 1295 CAS.
- W. H. Chuah, W. L. Zhang, H. J. Choi and Y. Seo, Macromolecules, 2015, 48, 7311–7319 CrossRef CAS.
- R. Sheng, G. A. Flores and J. Liu, J. Magn. Magn. Mater., 1999, 194, 167–175 CrossRef CAS.
- J. M. Ginder and L. C. Davis, Appl. Phys. Lett., 1994, 65, 3410 CrossRef CAS.
- J. M. Ginder, L. C. Davis and L. D. Elie, Int. J. Mod. Phys. B, 1996, 10, 3293 CrossRef CAS.
- F. F. Fang, J. H. Kim and H. J. Choi, Polymer, 2009, 47, 2290–2293 CrossRef.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 1565–1573 CrossRef CAS PubMed.
- V. Valdiglesias, G. Kilic, C. Costa, N. Fernandez-Bertolez, E. Pasaro, J. P. Teixeira and B. Laffon, Environ. Mol. Mutagen., 2015, 56, 125–148 CrossRef CAS PubMed.
- E. Allemann and R. Gurny, Eur. J. Pharm. Biopharm., 1993, 39, 173–191 CAS.
- K. P. Garcia, K. Zachsler, L. Barbaro, J. A. Barreto, W. O'Malley, L. Spiccia, H. Stephan and B. Graham, Small, 2014, 10, 2516–2529 CrossRef CAS PubMed.
- M. De Nicola, D. M. Gattia, E. Traversa and L. Ghibelli, J. Nanopart. Res., 2013, 15, 1711 CrossRef.
- F. Caputo, M. De Nicola and L. Ghibelli, Biochem. Pharmacol., 2014, 92, 112–130 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |