Carbon nanotube/polyimide bilayer thin films with high structural stability, optical transparency, and electric heating performance
Abstract
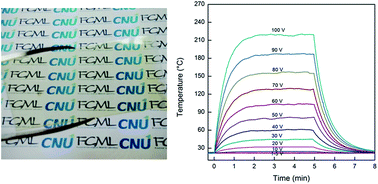
Structurally stable and optically transparent multiwalled carbon nanotube/polyimide (MWCNT/PI) bilayer thin films with different MWCNT thicknesses of 56–155 nm are manufactured by a facile spin-coating of MWCNT aqueous solution and poly(amic acid) (PAA) solution on a glass substrate, followed by thermal treatment for imidization. For this purpose, PAA as a PI precursor is synthesized by the reaction of pyromellitic dianhydride and 4,4′-diaminodiphenylether. The MWCNT layer thickness in the bilayer films is controlled by the cycle number of the spin-coating process of the MWCNT aqueous solution on the glass substrate. SEM images of the bilayer films reveal that neat MWCNT layers are uniformly coated on glass substrates and they are covered well with a PI layer. Accordingly, the MWCNT/PI bilayer films are mechanically and structurally stable owing to the presence of a PI layer on the MWCNT layer, compared to neat MWCNT films on glass substrates. With the increase of the MWCNT layer thickness in the bilayer films from 56 nm to 155 nm, the sheet resistance decreases from ∼1.97 × 105 Ω sq−1 to ∼3.89 × 103 Ω sq−1 and the optical transparency at 550 nm wavelength also decreases from ∼78% to ∼52%. The MWCNT/PI bilayer thin films exhibit a high electric heating performance in view of the rapid heating/cooling response time of 13.8–16.8 s, high electric power efficiency of 11.5–14.8 mW per °C, and high steady-state maximum temperatures up to 219 °C as a function of the applied voltage of 20–100 V.

 Please wait while we load your content...
Please wait while we load your content...