A novel route to fabricate high-density graphene assemblies for high-volumetric-performance supercapacitors: effect of cation pre-intercalation†
Abstract
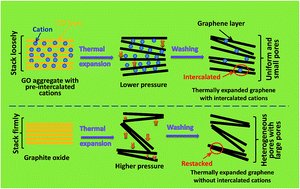
A simple procedure of cation pre-intercalation is incorporated with the ordinary graphene oxide thermal reduction method, readily producing graphene assemblies with a compact laminated structure and enhanced density, which exhibit anomalously high gravimetric capacitances and outstanding volumetric performances (a volumetric energy density of 26 W h L−1 in the aqueous electrolyte), primarily due to the contribution of ultramicropores.

 Please wait while we load your content...
Please wait while we load your content...