DOI:
10.1039/C6RA03878F
(Paper)
RSC Adv., 2016,
6, 36459-36466
A sensitive electrochemical Hg2+ ions sensor based on polypyrrole coated nanospherical platinum
Received
11th February 2016
, Accepted 7th April 2016
First published on 7th April 2016
Abstract
We report the synthesis and characterization of polypyrrole coated on nanospherical platinum (Pt/PPy NSs) composites for the detection of mercury ions. The synthesis was completed via the direct reduction of potassium tetrachloro platinate(II) aqueous solution in the presence of pyrrole monomers in NaOH. X-ray diffraction and field emission scanning electron microscopy results indicated that the Pt cations were completely reduced to Pt with the concurrent formation of Pt/PPy NSs nanospherical morphology, respectively. The electrochemical properties of Pt/PPy NSs electrode were studied by cyclic voltammetry, differential pulse voltammetry (DPV) and electrochemical impedance spectroscopy. From the DPV results, a linear working range for the concentration of mercury ions between 5 to 500 nM with LOD 0.27 nM (S/N = 3) was obtained. The sensitivity of this linear segment is 1.239 μA nM−1 cm−2. The interference from Ag+, Fe2+, Mn2+, K+, Pd2+, Cu2+, Ni2+, Pb2+, Sn2+ and Zn2+ was negligible, thus is a bright prospect for the electrochemical detection of Hg(II).
1 Introduction
The detection of mercury cations is of paramount importance, due to its toxicity in binding with sulfur-containing proteins and enzymes.1 Due to these effects, the development of a rapid, simple and economical sensor for the detection of mercury cations is in high demand in clinical diagnosis, industrial, food and environmental applications.2,3 Several techniques have been developed for the detection of mercury cations such as atomic absorption spectrophotometry (AAS),4 inductively coupled plasma-atomic emission spectroscopy (ICPAES),5 inductively coupled plasma-mass spectrometry (ICP-MS),5 X-ray fluorescence (XRF)6 and neutron activation analysis (NAA),7 but such applications are limited by their expensive cost. Electrochemical techniques have been widely used for the detection of Hg(II), due to the high sensitivity, low cost and ease of miniaturization. E. Wang et al.8 reported a three-dimensional fibril-like carbon fiber mat electrode (CFME) decorated with Au nanoparticles (AuNPs). They showed that the highly porous feature of CFME and the high affinity of AuNPs could increase the sensitivity of mercury detection. The use of conducting polymers such as polypyrrole (PPy), polythiophene and polyaniline is another method of electrode fabrication for the detection of Hg2+. It is notable that small perturbations at the surface or in the bulk of conducting polymers could trigger strong changes in the electroactivity, which can be probed by amperometry or potentiometry.
PPy is a widely used conducting polymer due to its good electrical conductivity and environmental stability.9,10 The composite of metal or metal oxide/PPy has better electronic properties compared to the pure material.11 Recent reports demonstrated that the composites of electroactive polymers with noble metal or metal oxide nanoparticles are good candidates for the detection of different types of analytes.12–14 Since the size of the composites may influence the sensitivity of detection of the analytes,15,16 and due to the unique advantages of nanosize materials, many researchers are developing facile synthetic methods for the production of nanocomposites of electroactive polymers with noble metals or metal oxides nanoparticles. One of the advantages of the combination of electroactive polymers and noble metals nanoparticles is the increase in the electrical conductivity, compared to the pure materials.17,18 Moreover, the synthesis of electroactive polymers in the presence of metal nanoparticles could increase the available surface area, as the nanoparticles and electroactive polymers could function as the core and the shell, respectively. With the increase of the surface area, high surface reaction activity, high catalytic efficiency and strong adsorption ability are useful for improving the sensor stability and sensitivity.19–21
In this work, we have developed a facile method for the preparation of polypyrrole coated platinum nanospherical (Pt/PPy NSs) composites via the direct reduction of platinum cations in the presence of pyrrole monomers. The application and performance of these new nanocomposites toward Hg2+ sensing are also investigated.
2 Experimental methods
2.1 Synthesis of polypyrrole coated palatinate nanospherical (Pt/PPy NSs) composites
All chemicals such as potassium tetrachloro-platinate(II), 99.9% metals basis, dimethyl formamide for molecular biology ≥99% (DMF), potassium ferricyanide (K3Fe(CN)6, 99.2%), ferric chloride, Na2HPO4 and NaH2PO4 were procured from Sigma-Aldrich (St. Louis, Mo, USA), while potassium ferrocyanide K4Fe(CN)6·3H2O was procured from Fischer Scientific (Shah Alam, Selangor, Malaysia). The synthesis of Pt/PPy NSs can be divided into three steps. First, 1 mL of 0.1 M potassium tetrachloro-platinate(II) solution was added into 30 mL 7 M NaOH solution in a reaction vessel; the mixture was kept at room temperature with continuous stirring at 500 rpm and a homogenous mixture was observed after 30 minutes. Second, 0.5 mL pyrrole monomer was added into the mixture and turned brown in color. The mixture was stirred for another 30 min to complete the redox reaction between the pyrrole monomers and Pt cations. Third, 0.01 mL hydrazine monohydrate was added into the reaction mixture and the temperature was increased to 60 °C at 1.5 °C min−1 and this process was sustained for another 60 minutes. Upon completion of the reaction, the mixture was centrifuged at 4000 rpm for 10 minutes to separate the Pt/PPy NSs from the mixture, followed by drying in a vacuum oven at 50 °C for 12 h. Moreover, pure PPy was synthesized as control material via chemical polymerization, by dissolving 0.1 mol ferric chloride in 150 mL distilled water in a reaction vessel and continuously stirred at 800 rpm with a mechanical stirrer. A solution of 0.05 mol distilled pyrrole monomer dissolved in 50 mL water was added drop-wise into the ferric chloride solution. The stirring was continued for 2 h at room temperature to complete the polymerization. The PPy was filtered and washed with distilled water repeatedly and dried in a vacuum oven at 40 °C for 24 h.
2.2 Electrode preparation
The synthesized Pt/PPy NSs (1 mg) was added in DMF (1 mL) and sonicated for 10 minutes to obtain a brown homogenous suspension. Then, 5 μL of the homogenous mixture was drop-casted onto the surface of a polished glassy carbon electrode (GCE) (polished with 0.05 μm alumina slurry using Buehler polishing kit), cleaned and dried at room temperature. The current density was calculated from the active area of GCE (0.07 cm2) which was covered by the synthesized Pt/PPy NSs.
2.3 Instrumentation and characterizations
Field emission scanning electron microscopy (FESEM, Quanta 200F) and energy dispersive X-ray (EDX) spectroscopy were used to investigate the morphology and weight percentage of the Pt/PPy NSs. The structure and phase composition of the prepared Pt/PPy NSs were characterized by X-ray diffraction (Siemens D5000) using CuKα radiation. The infrared spectrum was recorded at room temperature on a Spectrum 400 (FT-IR/FT-FIR spectrometer). Electrochemical impedance spectroscopy (EIS) measurements were performed by a potentiostat/galvanostat from Autolab, PGSTAT-302N (Ecochemie, Netherlands), controlled by a USB IF030 (MetrohmAutolab) interface card with the FRA.EXE software (version: 409.007, distributor: Metrohm Malaysia) installed in a PC. The quality of the fitting of the equivalent circuit was judged, first, by the x2-value (i.e. the sum of the square of the difference between the theoretical and experiment points) and second, by limiting the relative error in the value of each element in the equivalent circuit to 5%. The electrochemical cell was a three-electrode system where the modified GCE was the working electrode. A platinum foil and a saturated calomel electrode (SCE) were the counter and reference electrodes, respectively. The electrolyte for EIS measurement was 1 mM Fe(CN)63−/4− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution with 0.1 M KCl supporting electrolyte (K3Fe(CN)6, 99.2% and K4Fe(CN)6·3H2O).
1) solution with 0.1 M KCl supporting electrolyte (K3Fe(CN)6, 99.2% and K4Fe(CN)6·3H2O).
3 Results and discussions
3.1 Materials characterizations
The nanospherical morphology of the Pt/PPy NSs can be confirmed by the FESEM images (Fig. 1(a) and (b)) which show that the nanospheres are bundled by the PPy. The nanospherical structure is clearly confirmed from a higher magnification of the FESEM image in Fig. 1(b). The size of each nanospheres shows the existence of a large surface area of the Pt/PPy NSs, which can be utilized as a catalyst for the Hg2+ detection. The weight percentage of Pt/PPy NSs was determined by EDX and is shown in Fig. 1(c). The EDX results show the existence of C (from PPy) and Pt (from reduced Pt(II)). The weight percentage of each element is given in Table 1. The existence of carbon in the EDX spectrum of Pt/PPy NSs confirms the presence of PPy as a coating in the Pt/PPy NSs in Fig. 1(c).
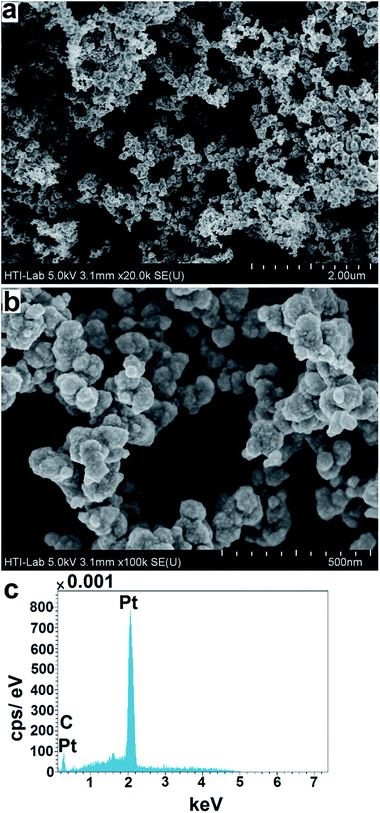 |
| | Fig. 1 (a) FESEM images of the Pt/PPy NSs (b) a higher magnification image of (a). (c) EDX of Pt/PPy NSs. | |
Table 1 EDX data of the Pt/PPy NSs
| Elements |
C |
Pt |
| W% |
2.806 ± 0.321 |
97.193 ± 0.321 |
The XRD pattern of Pt/PPy NSs and PPy are shown in Fig. 2(a) and (b) respectively. The intensity of the (111), (200) and (220) peaks in the diffractogram are related to the Pt/PPy NSs (Ref. code: 00-001-1194). The XRD result of Pt/PPy NSs shows a broad amorphous diffraction peak between 2θ = 5–20°, which is related to the scattering of the bare polymer chains at the interplanar spacing.22,23 The XRD of PPy shows a broad amorphous diffraction peak at 2θ = 10–40° (Fig. 2(b)). The broad diffraction peak of PPy in the Pt/PPy NSs is replaced with the peak (111) of Pt, indicating that the crystallinity of PPy is much lower than the Pt/PPy NSs. Based on the XRD result, we can conclude that the Pt2+ was converted to Pt metal in the presence of pyrrole monomers during the synthesis of Pt/PPy NSs. Moreover, from the XRD and FESEM results, it can be concluded that the polymerized PPy encapsulates the surface of Pt nanoparticles, forming a nucleus for further growth. These results can be explained by the Ostwald ripening process,24–26 which acts as a barrier against the corrosive NaOH environment.27
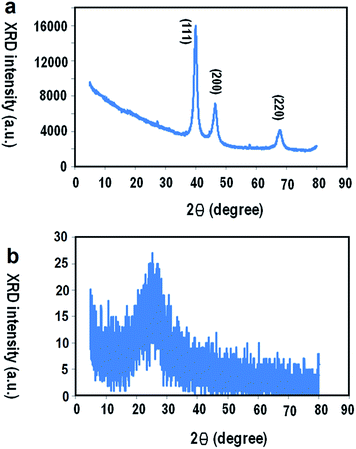 |
| | Fig. 2 XRD patterns of: (a) the Pt/PPy NSs, (b) synthesized PPy in the present of FeCl3. | |
Fig. 3(a) and (b) is the FTIR spectrum of the Pt/PPy NSs and PPy (the control spectrum, synthesized in the presence of FeCl3 solution), respectively. The polymerization of pyrrole monomers in the presence of Pt2+ can be confirmed by the presence of the following peaks in Fig. 3(a). First, the peak at 3350 cm−1 is related to the N–H bond. Second, the peak at 1670 cm−1 is related to the C–N–C bond or the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, which suggests that an over-oxidation of the pyrrole monomers had occurred during the PPy polymerization. The over-oxidation of PPy takes place through the apparition of the C–OH and C
O group, which suggests that an over-oxidation of the pyrrole monomers had occurred during the PPy polymerization. The over-oxidation of PPy takes place through the apparition of the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups in the polymer backbone, as well as the formation of CO2 at sufficiently positive potentials or in the presence of a strong oxidizer.28 Third, the peak at 1389 cm−1 is attributed to a typical PPy ring vibration. Moreover, the peaks at 1180 and 1080 cm−1 are due to the C–N and C–H groups in the composite spectrum, respectively. All of the described peaks are observed in the FTIR of PPy as the control spectrum, therefore it clearly confirms the polymerization of the pyrrole monomers and the over-oxidation of PPy in the presence of Pt2+ cations. On the other hand, the Pt2+ acts as an oxidizing agent and is reduced to metallic Pt during the polymerization of pyrrole.
O functional groups in the polymer backbone, as well as the formation of CO2 at sufficiently positive potentials or in the presence of a strong oxidizer.28 Third, the peak at 1389 cm−1 is attributed to a typical PPy ring vibration. Moreover, the peaks at 1180 and 1080 cm−1 are due to the C–N and C–H groups in the composite spectrum, respectively. All of the described peaks are observed in the FTIR of PPy as the control spectrum, therefore it clearly confirms the polymerization of the pyrrole monomers and the over-oxidation of PPy in the presence of Pt2+ cations. On the other hand, the Pt2+ acts as an oxidizing agent and is reduced to metallic Pt during the polymerization of pyrrole.
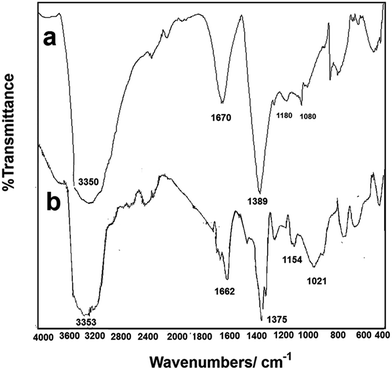 |
| | Fig. 3 FTIR of: (a) Pt/PPy NSs, (b) synthesized PPy in the present of FeCl3. | |
3.2 EIS results
The interfacial properties of the GCE electrodes modified with Pt/PPy NSs were studied by EIS. The Nyquist plots of the Pt/PPy NSs/GCE (1), PPy/GCE (2), bare (3) and their equivalents circuits in 1 mM Fe(CN)63−/4− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.1 M KCl supporting electrolyte are shown in Fig. 4. A comparison between the Nyquist plots of the Pt/PPy NSs GCE (1), PPy/GCE (2) and bare GCE (3) clearly confirms the effect of Pt/PPy NSs and PPy on the charge transfer resistance. The decrease in the charge transfer resistance from GCE to Pt/PPy NSs can be attributed to the conductivity of PPy and Pt. The presence of a peak related to the over-oxidation of polymerized PPy in the presence of Pt2+ in the FTIR spectrum, confirms that this phenomenon could decrease the conductivity of PPy, but the existence of Pt NPs could decrease the over-oxidation effect on the PPy conductivity. This effect can be confirmed from the semicircle diameter in the Nyquist plots of PPy/GCE and Pt/PPy NSs; together with the Rct (charge transfer resistance) obtained from the simulation of the EIS results of Pt/PPy NSs/GCE, PPy/GCE and the bare GCE (Table 2). The Rct is related to the primary resistance against electron transfer. The Nyquist plots of Pt/PPy NSs/GCE, PPy/GCE and bare GCE show a “depressed semi-circle” with the center of the semi-circle below the Zre axis, which is due to the deviation from the double-layer capacitance. The value of “n” is related to the slope of the log
1) with 0.1 M KCl supporting electrolyte are shown in Fig. 4. A comparison between the Nyquist plots of the Pt/PPy NSs GCE (1), PPy/GCE (2) and bare GCE (3) clearly confirms the effect of Pt/PPy NSs and PPy on the charge transfer resistance. The decrease in the charge transfer resistance from GCE to Pt/PPy NSs can be attributed to the conductivity of PPy and Pt. The presence of a peak related to the over-oxidation of polymerized PPy in the presence of Pt2+ in the FTIR spectrum, confirms that this phenomenon could decrease the conductivity of PPy, but the existence of Pt NPs could decrease the over-oxidation effect on the PPy conductivity. This effect can be confirmed from the semicircle diameter in the Nyquist plots of PPy/GCE and Pt/PPy NSs; together with the Rct (charge transfer resistance) obtained from the simulation of the EIS results of Pt/PPy NSs/GCE, PPy/GCE and the bare GCE (Table 2). The Rct is related to the primary resistance against electron transfer. The Nyquist plots of Pt/PPy NSs/GCE, PPy/GCE and bare GCE show a “depressed semi-circle” with the center of the semi-circle below the Zre axis, which is due to the deviation from the double-layer capacitance. The value of “n” is related to the slope of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z vs. log
Z vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f, in the Bode diagram. The decrease of “n” from unity is due to the porous and inhomogeneous surface. The increase of CPE of the Pt/PPy NSs/GCE compared to PPy/GCE (Table 2) confirms the existence of pores in the composite but the decrease of “n” is due to the increase of the surface roughness. Based on our results, it can be concluded that the polymerization of pyrrole monomers in the presence of Pt2+ has some advantages and disadvantages for the detection of Hg2+. The FESEM results show that Pt can play a role as the core and the pyrrole monomers (as the shell) are polymerized on the surface of the Pt nanoparticles, and this phenomenon increases the available surface area of PPy for the reaction with Hg2+. The second advantage is that the conductivity of the nanocomposite is greater than the pure PPy. Third, from this core–shell structure, numerous types of pores can be present in the nanocomposite. The main disadvantage is that the decrease in the surface roughness can decrease the available surface area for the reaction with the analyte.
f, in the Bode diagram. The decrease of “n” from unity is due to the porous and inhomogeneous surface. The increase of CPE of the Pt/PPy NSs/GCE compared to PPy/GCE (Table 2) confirms the existence of pores in the composite but the decrease of “n” is due to the increase of the surface roughness. Based on our results, it can be concluded that the polymerization of pyrrole monomers in the presence of Pt2+ has some advantages and disadvantages for the detection of Hg2+. The FESEM results show that Pt can play a role as the core and the pyrrole monomers (as the shell) are polymerized on the surface of the Pt nanoparticles, and this phenomenon increases the available surface area of PPy for the reaction with Hg2+. The second advantage is that the conductivity of the nanocomposite is greater than the pure PPy. Third, from this core–shell structure, numerous types of pores can be present in the nanocomposite. The main disadvantage is that the decrease in the surface roughness can decrease the available surface area for the reaction with the analyte.
 |
| | Fig. 4 Nyquist plots of the Pt/PPy NSs/GCE (1), PPy/GCE (2), bare (3) and their equivalents circuits in 1 mM Fe(CN)63−/4− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.1 M KCl supporting electrolyte. 1) with 0.1 M KCl supporting electrolyte. | |
Table 2 Electrochemical parameters obtained from the simulation of the EIS results of the Pt/PPy NSs/GCE in 1 mM Fe(CN)63−/4− (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.1 M KCl supporting electrolyte
1) with 0.1 M KCl supporting electrolyte
| Electrode |
R1 (Rs) ohm cm2 |
R2 (Rct) ohm cm2 |
Q1Y° (mohm−1 sn cm−2) |
n1 |
WY° (mS s1/2 cm−2) |
| GCE |
2.27 |
3950.10 |
0.0013 |
0.9902 |
0.1086 |
| Pt NSs-PPy |
2.81 |
177.12 |
0.1460 |
0.9262 |
2.8370 |
| PPy/GCE |
2.93 |
898.21 |
0.0548 |
0.7884 |
— |
3.3 Electrochemical behavior of Hg2+
The cyclic voltammograms (CVs) of Pt/PPy NSs/GCE (1), PPy/GCE (2) and bare GCE (4) in the presence of 10 μM Hg2+ in a mixture of 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 as supporting electrolyte, at pH 6.9 are shown in Fig. 5(a). The same conditions were employed for the comparison of the CV of Pt/PPy NSs/GCE (2) in the absence of Hg2+ from −0.8 V to 1.2 V (vs. SCE) at 50 mV s−1. Taking into account the fact that the redox potential of Hg2+ (E0 (2Hg2+/Hg22+) = +0.67 V (vs. SCE)) is considerably higher than Eredox of PPy, therefore the polymer is supposed to be partially oxidized by means of the Hg2+ ions (trapped in the polymer matrix after the adsorption step) when the latter is reduced to Hg22+, to form a complex between the Hg22+ and PPy at the surface of the electrode.29–31 The anodic peak located at 0.57 V in the CV of Pt/PPy NSs/GCE (1) in the presence of 10 mM Hg2+ is attributed to the electro-oxidation of the as-formed Hg22+ (in the complex) to the Hg2+ ions. The high sensitivity of the nanocomposite in the detection of Hg2+ is due to the high conductivity of the Pt nanoparticles which facilitates faster electron transfer in the polymer backbone, thus promoting the favorable reduction to Hg22+. Moreover, the cathodic peak at around +0.15 V is related to the reduction of Hg2+ to Hg22+ and the reduction of oxidized PPy from the reaction with Hg2+. This interpretation can be confirmed form the comparison of CVs of Pt/PPy NSs in the presence (1) and absence (2) of Hg2+. The results show a small shift and the increase of the size of the reduction peak in the presence of Hg2+. This peak can be seen in the CV of PPy/GCE (2) and is related to the reduction of the oxidized PPy. Theoretically, mercury like other 3d metal, such as copper, cadmium and zinc have poorly defined reduction waves, but show sharp and intense re-oxidation stripping waves.32 The cathodic peak at 0.7 V is related to the reduction of the over-oxidized PPy in the CVs of Pt/PPy NSs, in the absence and presence of Hg2+. In summary, after the adsorption step, due to the chelating complexation (a common feature of M2+ ions), the selective electrochemical detection of Hg2+ can occur owing to its characteristic value of E0 (Hg2+/Hg22+) which triggers the reduction of Hg2+ → Hg22+, driven chemically (with the help of PPy) or electrochemically (under more negative potential bias). Fig. 5(b) shows the reproducibility of the synthesized Pt/PPy in the detection of Hg2+. We synthesized Pt/PPy three times and checked the detection of Hg2+ separately. The result shows an acceptable reproducibility of the synthesized Pt/PPy.
 |
| | Fig. 5 (a) Cyclic voltammograms (CVs) of Pt/PPy NSs/GCE (1) and PPy/GCE (2), bare GCE (4) in the presence of 10 μM Hg2+ in 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 as supporting electrolyte, at pH 6.9. The CV of Pt/PPy NSs/GCE (2) in the absence of Hg2+ in 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 as supporting electrolyte at pH 6.9 (scan rate: 50 mV s−1). (b) Reproducibility of synthesized Pt/PPy to detection of Hg2+. | |
3.4 Effects of the pH of the solution
The effect of pH on the electrochemical response of the Pt/PPy NSs/GCE upon the addition of 10 μM Hg2+ was investigated using CV. The change in the peak current with pH (pH range of 6.3–7.2) is shown in Fig. 6. Since this electrode is utilized in the detection of Hg2+ in ground water and pipe water, where the pH is close to 7, we only considered a narrow range of pH around 7. It can be observed that the anodic peak current increases with pH until pH 6.9. The reasons can be explained as follows. With the decrease of pH and increase in the concentration of H+, the competition between Hg2+ and H+ for the reactive sites in PPy is intensified. This is notable where the first step in the detection of Hg2+ is the adsorption and trapping of the Hg2+ in the PPy matrix. On the other hand, the current density decreases with the increase of pH > 7, due to the interaction of Hg2+ with OH−. Therefore, the buffering at pH 6.9 which is close to the pH of pure water is used in the rest of the work.
 |
| | Fig. 6 The change in peak current with pH (pH range of 6.5–7.4). | |
3.5 Effects of scan rate
Fig. 7(a) shows the CVs of Pt/PPy NSs/GCE at different scan rates in the presence of 10 μM Hg2+ in 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 at pH 6.9. The increase of the oxidation and reduction peak currents with the scan rate shows that the electrode reaction of the immobilized Pt/PPy NSs redox couple is a surface-confined electrochemical process. The linear regression equation can be expressed as Ipa = 0.03υ (mV s−1) + 0.40 (R2 = 0.996) and Ipc = −0.03 (mV s−1) + 0.50 (R2 = 0.993) (Fig. 7(b)).
 |
| | Fig. 7 (a) CVs of Pt/PPy NSs/GCE at different scan rates (10, 20, 30, 40, 50, 60, 70, 80, 90, 100 and 200 mV s−1) in the presence of 10 μM Hg2+ in 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 as supporting electrolyte, at pH 6.9. (b) Linear plots of the oxidation and reduction currents versus scan rate. | |
3.6 Determination of Hg2+ using differential pulse voltammetry
A typical amperometric response of the Pt/PPy NSs/GCE to Hg2+ concentration changes was studied with differential pulse voltammetry (DPV) 10 μM Hg2+ in 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 at pH 6.9. Fig. 8(a) shows the dependence of the anodic peak current (Ipa) on the Hg2+ concentration. The sensor was calibrated three times and the standard deviations were calculated. The calibration curve for the Pt/PPy NSs/GCE shows a linear segment: from 5 to 500 nM with a linear regression equation of Ipa = 0.001 (mA μM−1 cm−2) + 0.024 (R2 = 0.996) (Fig. 8(b)). The limit of detection (LOD) (S/N = 3) and the limit of quantification (LOQ) (S/N = 10) of the Pt/PPy NSs/GCE were calculated from the following equations:33where SB is the standard deviation of the blank solution and b is the slope of the analytical curve, as shown in Fig. 8(b). The estimated LOD (S/N = 3), LOQ (S/N = 10) for the linear segment is 0.277 nM, and 0.924 nM, respectively. In addition, the sensitivity of the linear segment is 1.239 μA nM−1 cm−2. The increase of the response of Pt/PPy NCs/GCE toward the Hg2+ concentration can be explained by the following reasons: first, the role of the Pt nanoparticles is to increase the conductivity of the composites and to decrease of over-oxidation effect due to the polymer. Second, the Pt nanoparticles are the core and therefore decrease the size of the PPy shell. Third, the size decrease of the PPy increases the total surface area of the polymer; this phenomenon promotes the interaction of PPy with the Hg2+ ions resulting in more adsorption and entrapment of Hg2+ into the polymer matrix.
 |
| | Fig. 8 (a) The DPV curves of different concentration of Hg2+ in 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 as supporting electrolyte (5, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 200, 300, 400 and 500 nM) at pH 6.9. (b) The calibration curve. Error bars represent ± standard deviation. | |
3.7 Interference effects and recovery experiment
The selectivity of the sensor was determined by investigating it with the presence of alkaline earth metal ion (K+) and transition metal ions (Ag+, Cu2+, Cu2+, Fe2+, Mn2+, Ni2+, Pb2+, Pd2+, Sn2+, Zn2+). Fig. 9 shows the electrochemical response of Hg2+ in the presence of a 100-fold individual species in the solution with respect to Hg2+. Among the various metal ions studied, Hg2+ shows the highest signal gain change compared to the other metal ions. Likewise, the sensor's response to Hg2+ is unaffected by the presence of the mixture of metal ions.
 |
| | Fig. 9 The selectivity of the sensor by investigating it with alkaline earth metal ion (K+) and transition metal ions (Ag+, Cu2+, Cu2+, Fe2+, Mn2+, Ni2+, Pb2+, Pd2+, Sn2+, Zn2+). The electrochemical response of Hg2+ in the presence of 100-fold individual species in the solution with respect to Hg2+. The illustrated error bars represent the standard deviations of measurements taken from at least three independent experiments. | |
For analytical applications, a recovery experiment was also carried out to examine the prospective use of the Pt/PPy NSs/GCE. Three different concentrations of Hg2+ (50, 200 and 400 nM) were added in a mixture of 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 at pH 6.9. The Hg2+ was detected by the renewed electrode with acceptable relative standard deviation (RSD), which was estimated from 6 parallel measurements. This result shows that the prepared Pt/PPy NSs/GCE sensor could be applied repeatedly without a significant decrease in its accumulation capacity for Hg2+, with high reproducibility and regeneration ability (Table 3).
Table 3 The detection of Hg2+ concentration in test samples (results based on six replicate determinations per sample)
| Sample |
Added (nM) |
Found (nM) |
RSD% |
Recovery% |
| 1 |
50 |
49.423 |
3.305 |
98.84 |
| 2 |
200 |
199.571 |
2.338 |
99.78 |
| 3 |
500 |
497.521 |
1.667 |
99.50 |
3.8 Analysis of real samples
The analytical application of the Pt/PPy NSs/GCE electrode was tested with ground water and pipe water in University of Malaya. Linear correlations (n = 3) between the peak current and the corresponding concentrations of Hg2+ were attained by using samples spiked at different concentration levels. The LODs for Hg2+ in the water samples, calculated from the analyte concentration, where the peak current is three times (S/N = 3), were 1.825 and 1.129 nM, for the ground water and pipe water, respectively (Table 4).
Table 4 Analysis of real samples
| Sample |
Linear range (nM) |
R% |
LOD (nM) |
| Pipe water |
10–100 |
99.1 |
1.125 |
| Ground water |
10–100 |
98.7 |
1.825 |
Table 5 shows that the LOD of Hg2+ for Pt/PPy NSs/GCE is lower compared to thiol-functionalized AgNPs,34 quantum dot/DNA/AuNPs ensemble,35 fluorescently labeled single-stranded DNA probe36 and the present result is comparable to AuNPs and thymine-rich hairpin DNA probes.37
Table 5 A summary and comparison of the estimated LOD values from the present work with previous reports
| Type of electrode |
Detection method |
Sensitivity |
Performance LOD/nM |
Linear range/nM |
Reference |
| Thiol-functionalized AgNPs |
Surface-enhanced Raman scattering |
— |
2.4 |
10–2000 |
34 |
| Quantum dot/DNA/AuNPs ensemble |
Fluorometry |
Fluorescence quenching efficiency as a function of the Hg2+ concentration: 0.007 F nM−1 |
2 |
2–60 |
35 |
| Fluorescently labeled single-stranded DNA probe and carbon nanoparticles |
Fluorometry |
1.85 μA nM−1 |
10 |
0–250 |
36 |
| AuNPs and thymine-rich hairpin DNA probes |
Colorimetry |
— |
0.1 |
0.1–100 |
37 |
| Coordination of Hg2+ to AuNPs associated nitrotriazole |
Colorimetry |
— |
7 |
10–500 |
38 |
| Thiolated ferrocene (Fc)-tagged DNA oligomer |
Fluorometry |
— |
100 |
10–2000 |
39 |
| Au-MPA-HFHAHFAF peptide modified electrode |
Electrochemistry |
0.5 μA μM−1 |
9.5 |
0.25–0.81 |
40 |
| Pt/PPy NSs (nanospherical) |
Electrochemistry |
1.239 μA nM−1 |
0.27 |
5–500 |
This work |
4 Conclusions
Polypyrrole coated platinum nanospherical (Pt/PPy NSs) composites were synthesized by a direct reduction of Pt2+ in the presence of pyrrole monomers in aqueous solution. The results demonstrated that the surface modification of the glassy carbon electrode with Pt/PPy NSs resulted in a superior electrocatalytic activity toward the electro-oxidation of 10 μM Hg2+ in a mixture of 0.33 M phosphate buffer solution, 0.5 M NaCl and 0.1 M LiClO4 at pH 6.9. The higher electroactivity of the Pt/PPy NSs was due to the following reasons. First, the FESEM results show that Pt can play the role of a core and pyrrole monomers (as the shell) can polymerize on the surface of the Pt nanoparticles. Due to this phenomenon, the size of the polymer decreased and the available surface area of the PPy increased. Second, the composite has larger conductivity than the PPy and third, the appearance of numerous types of pores in the composites. The higher sensitivity of the composite for the detection of Hg2+ is due to the higher conductivity of the Pt nanoparticles, which promotes faster electron transfer process along the polymer backbone, thus induced more favorable reduction to Hg22+. The estimated LOD (S/N = 3) and LOQ (S/N = 10) for the linear segment was 0.277 nM and 0.924 nM, respectively. In addition, the sensitivity of this linear segment was 1.239 μA nM−1 cm−2.
Acknowledgements
The authors wish to thank Mojdeh Yeganeh for valuable discussion. This research is supported by High Impact Research MoE Grant UM.C/625/1/HIR/MoE/SC/04 from the Ministry of Education Malaysia, PRGS grant PR002-2014A, GC001C-14SBS, RP038C-15HTM and University Malaya Centre for Ionic Liquids (UMCiL).
References
- P. B. Tchounwou, W. K. Ayensu, N. Ninashvili and D. Sutton, Environ. Toxicol., 2003, 18, 149 CrossRef CAS PubMed.
- F. Zahir, S. J. Rizwi, S. K. Haq and R. H. Khan, Environ. Toxicol. Pharmacol., 2005, 20, 351 CrossRef CAS PubMed.
- Z.-G. Liu and X.-J. Huang, Trends Anal. Chem., 2014, 60, 25 CrossRef CAS.
- E. M. Martinis, P. Berton, J. C. Altamirano, U. Hakala and R. G. Wuilloud, Talanta, 2010, 80, 2034 CrossRef CAS PubMed.
- H. Hsieh, W. Chang, Y. Hsieh and C. Wang, Talanta, 2009, 79, 183 CrossRef CAS PubMed.
- V. S. Hatzistavros and N. G. Kallithrakas-Kontos, Electrochim. Acta, 2014, 809, 25 CAS.
- K. K. Sivasankara Pillay, C. C. Thomas, Jr, J. A. Sondel and C. M. Hyche, Anal. Chem., 1971, 43, 1419 CrossRef.
- D. Li, J. Li, X. Jia and E. Wang, Electrochem. Commun., 2014, 42, 30 CrossRef CAS.
- X. Kang, J. Wang, H. Wu, I. A. Aksay, J. Liu and Y. Lin, Biosens. Bioelectron., 2009, 25, 901 CrossRef CAS PubMed.
- A. Ashrafi, M. A. Golozar and S. Mallakpour, Synth. Met., 2006, 156, 1280 CrossRef CAS.
- J. Zang and X. Li, J. Mater. Chem., 2011, 21, 10965 RSC.
- Y. Fan, J.-H. Liu, C.-P. Yang, M. Yu and P. Liu, Sens. Actuators, B, 2011, 157, 669 CrossRef CAS.
- Y. Luo, W. Lu, G. Chang, F. Liao and X. Sun, Electrochim. Acta, 2011, 56, 8371 CrossRef CAS.
- S. Ye and Y. Lu, J. Phys. Chem. C, 2008, 112, 8767 CAS.
- M. R. Mahmoudian, W. J. Basirun, Y. Alias and M. Ebadi, Electrochim. Acta, 2012, 72, 46 CrossRef CAS.
- G. Chang, Y. Luo, W. Lu, X. Qin, A. M. Asiri, A. O. Al-Youbi and X. Sun, Catal. Sci. Technol., 2011, 1, 1393 Search PubMed.
- H. G. Lemos, S. F. Santos and E. C. Venancio, Synth. Met., 2015, 203, 22 CrossRef CAS.
- P. K. Kalambate, R. A. Dar, S. P. Karna and A. K. Srivastava, J. Power Sources, 2015, 276, 262 CrossRef CAS.
- J. Q. Wang, S. Chua, F. Kong, L. L. Luo, Y. Wang and Z. G. Zou, Sens. Actuators, B, 2010, 150, 25 CrossRef CAS.
- J. S. Liu and X. Z. Du, J. Mater. Chem., 2011, 21, 6981 RSC.
- Z.-Q. Zhao, X. Chen, Q. Yang, J.-H. Liu and X.-J. Huang, Chem. Commun., 2012, 48, 2180 RSC.
- J. Ouyang and Y. Li, Polymer, 1997, 38, 3997 CrossRef CAS.
- R. E. Partch, S. G. Gangoli, E. Matijevic, W. Cai and S. Arajs, J. Colloid Interface Sci., 1991, 144, 27 CrossRef CAS.
- I. Seo, M. Pyo and G. Cho, Langmuir, 2002, 18, 7253 CrossRef CAS.
- W. C. Carter and A. R. Roosen, Phys. A, 1998, 261, 232 CrossRef.
- M. Sookhakian, Y. M. Amin, W. J. Basirun, M. T. Tajabadi and N. Kamarulzaman, J. Lumin., 2014, 145, 244 CrossRef CAS.
- M. B. Inoue, K. W. Nebesny and Q. Fernando, Synth. Met., 1990, 38, 205 CrossRef CAS.
- T. W. Lewis, G. G. Wallace, C. Y. Kim and D. Y. Kim, Synth. Met., 1997, 84, 403 CrossRef CAS.
- B. J. Patys, M. Skompska and K. Jackowska, J. Electroanal. Chem., 1997, 433, 41 CrossRef.
- J. Rutkowska, K. Kilian and K. Pyrzynska, Eur. Polym. J., 2008, 44, 2108 CrossRef CAS.
- K. Kilian and K. Pyrzynska, React. Funct. Polym., 2008, 68, 974 CrossRef CAS.
- R. Agaz, M. T. Sevilla and L. Hernandez, Anal. Chim. Acta, 1993, 15, 205 CrossRef.
- I. Krull and M. Swartz, LC–GC, 1998, vol. 16, p. 922 Search PubMed.
- Y. Chen, L. H. Wu, Y. H. Chen, N. Bi, X. Zheng, H. B. Qi, M. H. Qin, X. Liao, H. Q. Zhang and Y. Tian, Microchim. Acta, 2012, 177, 341 CrossRef CAS.
- A. M. Ashrafi and K. Vytřas, Talanta, 2011, 85, 2700 CrossRef CAS PubMed.
- X. C. Fu, X. Chen, Z. Guo, L. T. Kong, J. Wang, J. H. Liu and X. J. Huang, Electrochim. Acta, 2010, 56, 463 CrossRef CAS.
- V. Somerset, J. Leanera, R. Masonb, E. Iwuohac and A. Morrind, Electrochim. Acta, 2010, 55, 4240 CrossRef CAS.
- X. J. Chen, Y. B. Zu, H. Xie, A. M. Kemas and Z. Q. Gao, Analyst, 2011, 136, 1690 RSC.
- D. Han, Y.-R. Kim, J.-W. Oh, T. Hyun Kim, R. Kumar Mahajan, J. Seung Kim and H. Kim, Analyst, 2009, 134, 1857 RSC.
- N. Daud, N. A. Yusof and T. W. Tee, Int. J. Electrochem. Sci., 2011, 6, 2798 CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution with 0.1 M KCl supporting electrolyte (K3Fe(CN)6, 99.2% and K4Fe(CN)6·3H2O).
1) solution with 0.1 M KCl supporting electrolyte (K3Fe(CN)6, 99.2% and K4Fe(CN)6·3H2O).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, which suggests that an over-oxidation of the pyrrole monomers had occurred during the PPy polymerization. The over-oxidation of PPy takes place through the apparition of the C–OH and C
O group, which suggests that an over-oxidation of the pyrrole monomers had occurred during the PPy polymerization. The over-oxidation of PPy takes place through the apparition of the C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups in the polymer backbone, as well as the formation of CO2 at sufficiently positive potentials or in the presence of a strong oxidizer.28 Third, the peak at 1389 cm−1 is attributed to a typical PPy ring vibration. Moreover, the peaks at 1180 and 1080 cm−1 are due to the C–N and C–H groups in the composite spectrum, respectively. All of the described peaks are observed in the FTIR of PPy as the control spectrum, therefore it clearly confirms the polymerization of the pyrrole monomers and the over-oxidation of PPy in the presence of Pt2+ cations. On the other hand, the Pt2+ acts as an oxidizing agent and is reduced to metallic Pt during the polymerization of pyrrole.
O functional groups in the polymer backbone, as well as the formation of CO2 at sufficiently positive potentials or in the presence of a strong oxidizer.28 Third, the peak at 1389 cm−1 is attributed to a typical PPy ring vibration. Moreover, the peaks at 1180 and 1080 cm−1 are due to the C–N and C–H groups in the composite spectrum, respectively. All of the described peaks are observed in the FTIR of PPy as the control spectrum, therefore it clearly confirms the polymerization of the pyrrole monomers and the over-oxidation of PPy in the presence of Pt2+ cations. On the other hand, the Pt2+ acts as an oxidizing agent and is reduced to metallic Pt during the polymerization of pyrrole.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.1 M KCl supporting electrolyte are shown in Fig. 4. A comparison between the Nyquist plots of the Pt/PPy NSs GCE (1), PPy/GCE (2) and bare GCE (3) clearly confirms the effect of Pt/PPy NSs and PPy on the charge transfer resistance. The decrease in the charge transfer resistance from GCE to Pt/PPy NSs can be attributed to the conductivity of PPy and Pt. The presence of a peak related to the over-oxidation of polymerized PPy in the presence of Pt2+ in the FTIR spectrum, confirms that this phenomenon could decrease the conductivity of PPy, but the existence of Pt NPs could decrease the over-oxidation effect on the PPy conductivity. This effect can be confirmed from the semicircle diameter in the Nyquist plots of PPy/GCE and Pt/PPy NSs; together with the Rct (charge transfer resistance) obtained from the simulation of the EIS results of Pt/PPy NSs/GCE, PPy/GCE and the bare GCE (Table 2). The Rct is related to the primary resistance against electron transfer. The Nyquist plots of Pt/PPy NSs/GCE, PPy/GCE and bare GCE show a “depressed semi-circle” with the center of the semi-circle below the Zre axis, which is due to the deviation from the double-layer capacitance. The value of “n” is related to the slope of the log
1) with 0.1 M KCl supporting electrolyte are shown in Fig. 4. A comparison between the Nyquist plots of the Pt/PPy NSs GCE (1), PPy/GCE (2) and bare GCE (3) clearly confirms the effect of Pt/PPy NSs and PPy on the charge transfer resistance. The decrease in the charge transfer resistance from GCE to Pt/PPy NSs can be attributed to the conductivity of PPy and Pt. The presence of a peak related to the over-oxidation of polymerized PPy in the presence of Pt2+ in the FTIR spectrum, confirms that this phenomenon could decrease the conductivity of PPy, but the existence of Pt NPs could decrease the over-oxidation effect on the PPy conductivity. This effect can be confirmed from the semicircle diameter in the Nyquist plots of PPy/GCE and Pt/PPy NSs; together with the Rct (charge transfer resistance) obtained from the simulation of the EIS results of Pt/PPy NSs/GCE, PPy/GCE and the bare GCE (Table 2). The Rct is related to the primary resistance against electron transfer. The Nyquist plots of Pt/PPy NSs/GCE, PPy/GCE and bare GCE show a “depressed semi-circle” with the center of the semi-circle below the Zre axis, which is due to the deviation from the double-layer capacitance. The value of “n” is related to the slope of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z vs. log
Z vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f, in the Bode diagram. The decrease of “n” from unity is due to the porous and inhomogeneous surface. The increase of CPE of the Pt/PPy NSs/GCE compared to PPy/GCE (Table 2) confirms the existence of pores in the composite but the decrease of “n” is due to the increase of the surface roughness. Based on our results, it can be concluded that the polymerization of pyrrole monomers in the presence of Pt2+ has some advantages and disadvantages for the detection of Hg2+. The FESEM results show that Pt can play a role as the core and the pyrrole monomers (as the shell) are polymerized on the surface of the Pt nanoparticles, and this phenomenon increases the available surface area of PPy for the reaction with Hg2+. The second advantage is that the conductivity of the nanocomposite is greater than the pure PPy. Third, from this core–shell structure, numerous types of pores can be present in the nanocomposite. The main disadvantage is that the decrease in the surface roughness can decrease the available surface area for the reaction with the analyte.
f, in the Bode diagram. The decrease of “n” from unity is due to the porous and inhomogeneous surface. The increase of CPE of the Pt/PPy NSs/GCE compared to PPy/GCE (Table 2) confirms the existence of pores in the composite but the decrease of “n” is due to the increase of the surface roughness. Based on our results, it can be concluded that the polymerization of pyrrole monomers in the presence of Pt2+ has some advantages and disadvantages for the detection of Hg2+. The FESEM results show that Pt can play a role as the core and the pyrrole monomers (as the shell) are polymerized on the surface of the Pt nanoparticles, and this phenomenon increases the available surface area of PPy for the reaction with Hg2+. The second advantage is that the conductivity of the nanocomposite is greater than the pure PPy. Third, from this core–shell structure, numerous types of pores can be present in the nanocomposite. The main disadvantage is that the decrease in the surface roughness can decrease the available surface area for the reaction with the analyte.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.1 M KCl supporting electrolyte.
1) with 0.1 M KCl supporting electrolyte.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 0.1 M KCl supporting electrolyte
1) with 0.1 M KCl supporting electrolyte







