Chromatographic analysis of multicomponent mixture of vitamins B1, B6, B12, benfotiamine and diclofenac; part II: LC-tandem MS/MS method for simultaneous quantification of five components mixture in pharmaceutical formulations and human plasma†
Abstract
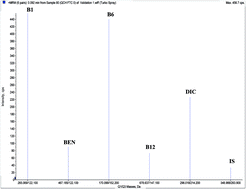
A novel high performance liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method was established for the simultaneous determination of multivitamins, namely thiamine hydrochloride (B1), pyridoxine (B6), cyanocobalamin (B12) and benfotiamine (BEN) and their co-formulated drug, diclofenac sodium (DIC) using torsemide as internal standard (IS). Chromatographic separation was accomplished on Shimadzu LC-20 AT Series HPLC, equipped with Sunfire C18 column (Waters) (50 × 4.6 mm, 5 μm) using methanol : 0.02 M ammonium acetate (80 : 20, v/v) pH 6.0 for B1, B12, BEN and DIC, while using methanol : acetonitrile : 0.01 M ammonium formate (80 : 10 : 10, v/v/v) pH 6.25 for B6 on separate runs at a flow rate of 1.0 mL min−1. Sample preparation involves extraction with methanol using 30 ng mL−1 of IS. Electrospray ionization (ESI) source was operated in the positive ion mode. The multiple reaction monitoring (MRM) mode on a triple quadrupole mass spectrometer was used to quantify the drugs utilizing the transitions of 265.07/122.10, 170.01/152.20, 678.64/147.01, 467.18/122.10, 296.02/214.20, 348.99/263.90 (m/z) for B1, B6, B12, BEN, DIC and IS, respectively. The proposed method was effectively applied for the analysis of laboratory prepared mixtures, in spiked human plasma as well as in their combined pharmaceutical formulations. A detailed analytical and bioanalytical validation was conducted in compliance with the FDA and ICH guidelines proving the method to be selective, linear, precise and accurate over the concentration ranges of 0.01–20.00, 0.02–50.00, 15.00–500.00, 5.00–500.00 and 10.00–500.00 ng mL−1 for the five compounds, in order. The simplicity and sensitivity of this method allows its use in the quality control of the cited drugs.

 Please wait while we load your content...
Please wait while we load your content...