Aptamer-functionalized P(NIPAM-AA) hydrogel fabricated one-dimensional photonic crystals (1DPCs) for colorimetric sensing
Hongyun Xuana,
Jiaoyu Rena,
Yanxi Zhua,
Bo Zhaob and
Liqin Ge*a
aState Key Laboratory of Bioelectronics, School of Biological Science and Medical Engineering, Southeast University, Nanjing 210096, P.R. China. E-mail: lqge@seu.edu.cn
bSchool of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210096, P.R. China
First published on 30th March 2016
Abstract
As heavy metals cannot be biodegraded and are easily accumulated in food chain organisms and so enter the human body, a simple detection method becomes particularly important for human health. A robust means for the visual detection of highly toxic mercury ion (Hg2+) is developed with aptamer-functionalized one-dimensional photonic crystals (1DPCs). 1DPCs consisting of TiO2 and poly(N-isopropylacrylamide-acrylic acid), P(NIPAM-AA), were successfully fabricated by a spin-coating technique, whose stopbands span the total visible range. When the 1DPCs were exposed to mercury ion solution, the specific binding of cross-linked single-stranded aptamers and heavy metal ions in the hydrogel network caused the hydrogel to shrink. At the same time, there was a homologous blue shift in the Bragg diffraction peak position of the TiO2/P(NIPAM-AA) 1DPCs. The shift value could be used to estimate the number of target ions quantitatively. It was found that the 1DPCs apta-sensor could screen a wide concentration range of heavy metal ions with selectivity and sensitivity. It is expected that the technology may also be widely used in screening a wide range of metal ions in drugs, food, and the environment.
Introduction
Heavy metal ions are accumulated in natural resources and converted to toxic chemicals in living organisms, which creates negative impacts on the environment and poses a risk to human health. Heavy metal ions can be harmful if taken too much through the following two ways.1–3 On the one hand, they make the normal stereo structure of enzymes distort and lose application activity as a result of losing physical activities,4–6 and excess heavy metal ions in the body strongly combine with the active groups of protein molecules, such as –SH, –NH2, –COOH, –OH. On the other hand, enzymes containing other metal ions replaced by some heavy metal ions would lose physiological activity, which causes great toxicity to human health and living things.7–12 Although the present methods of detection of heavy metal ions including atomic absorption spectrometry,13 inductively coupled plasma-mass spectrometry14,15 and X-ray fluorescence spectroscopy16 can generate more than sensitive analytical results, they generally require costly equipment, complicated and time-consuming experiments and high level of operator skills, and they play an important role in environmental monitoring and safety evaluation of aquatic food supplies.In order to overcome the limitations, many researchers have been directing their efforts into achieving simple, cheap and on-the-spot means of detection. It is well known that fluorophores17,18 and chromophores19,20 are comprehensive for detecting heavy metal ions, but only few of them have high selectivity and most are subject to the limitations of low water solubility, long response, and cross-reactivity with other coexisting metal ions. To improve selectivity, enzyme-based sensors depending on the control of a catalytic reaction are applied to heavy metal ion detection. Some problems in the structure of the sensors have come out, such as the enzyme reagent stability, high cost, and enzyme production of high difficulty, so we should look for novel sensors to screen heavy metal ions.21,22
Antibodies, like single-stranded DNA as aptamers, because of their innate merits of easy regeneration, simple production, target versatility, convenient storage and easy modification, are considered to be perfect recognition elements for sensor applications.23–25 Lately studies have mainly focused on how to make aptamers as sensing elements become novel sensors for detecting Hg2+ by the principle26–30 that Hg2+ can specifically interact with thymine bases to form thymine–Hg2+–thymine (T–Hg2+–T) compound.31 Most of the methods come down to various labeled fluorophores modifying the aptamer and signals are transduced via FRET or colorimetric methods to permit detection so that we need to develop a simple and visual detection method for aptamer-based heavy metal ion analysis.
Photonic crystals, first introduced by Eli Yablonovitch and Sajeev John in 1987,32,33 which can modulate light within a wavelength by photonic stopbands where light propagation is prohibited,34,35 as the change of dielectric constant is large enough and the change cycle is in accordance with the wavelength of light, are new-style structural materials and consist of orderly repeating and periodic structures of high and low dielectric constant causing the propagation of optical waves. Though photonic crystals can be divided into one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) photonic stopbands, photonic stopbands are the common characteristic.
Photonic crystals are applied in chemical and biological sensing, for example biotechnology fields,36 environmental monitoring,37 medical examination,38 and so on, because they possess novel and unique microstructures and optical properties. By using the principle of Bragg stacks and distributed Bragg mirrors, 1D photonic crystals (1DPCs) are made into two or more alternating high- and low-refractive-index materials,39 whose optical properties can be tuned by changing incident angles, periods and refractive index40 according to which photonic stopbands could be estimated from Bragg's law. Spin-coating is the cheapest and most convenient method to prepare 1DPCs by adjusting the period of 1DPCs based on controlling the solution concentration, the time of spin-coating and the rotational speed, compared with other methods such as sputtering, evaporation,41 sol–gel process,42 chemical vapour deposition43 and holographic polymerization.44 No matter from which viewpoint, 1DPCs are the simplest photonic crystals of all 2D photonic crystals and 3D photonic crystals so that many researchers have concentrated their efforts on the fabrication by spin-coating and application of functional 1DPCs in recent years.48,49
The position of the Bragg peak of 1DPCs can be calculated using the following formula:
 | (1) |
In our current work, for TiO2/P(NIPAM-AA) 1DPCs fabricated by the spin-coating technique, it turns out that 1DPCs with different stopbands can be obtained by controlling the spin-coating and incident angles. Then, a key consideration for using the 1DPCs sensor was the stimulating material, which should offer a common method for any specific target. And that is the reason why the aptamer-functionalized P(NIPAM-AA) that we introduced as a target-responsive aptamer swells or shrinks upon being stimulated which could lead to a change in the 1DPCs accompanied by a visual colour change because the specific binding aptamer with target ions changes the aptamer conformation and triggers shrinkage of P(NIPAM-AA) as the aptasensors were exposed to a solution of heavy metal ions. TiO2/P(NIPAM-AA) 1DPCs based on a corresponding blue shift in the Bragg diffraction peak position were applied for the quantitative estimation of Hg2+ ions.
Experimental
Materials
The Hg2+ aptamer (5′-NH2-(CH2)6-TTCTTTCTTCCCCTTGTTTGTT-(CH2)6-NH2-3′) was bought from Shanghai Sangon Biological Engineering Co. Ltd (Shanghai, China). Tetrabutyl titanate was obtained from Shanghai Ling Feng Chemical Reagent Co. Ltd. N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC), N-hydroxysuccinimide (NHS) and tris(hydroxymethyl)aminomethane (Tris) were purchased from Sigma-Aldrich (Shanghai, China). Poly(N-isopropylacrylamide-acrylic acid) hydrogel was synthesized by Southeast University (Nanjing, China). The silicon wafers were soaked in a compound of 98% H2SO4/30% H2O2 at a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 24 h, then washed with deionized water three times and dried with a N2 stream finally.
1 for 24 h, then washed with deionized water three times and dried with a N2 stream finally.
Synthesis and preparation of 1DPCs
In brief, 2 ml tetrabutyl titanate and 2 ml acetic acid were dissolved into 32 ml ethanol, which were added into a conical flask and stirred for five hours at room temperature.At first, the carboxyl and hydroxyl group-functionalized P(NIPAM-AA) were activated with EDC/NHS by soaking in 1 ml pH 5.5 PBS buffer solution containing 10 mg EDC and 2 mg NHS, then dripping the above mixture into 1 ml P(NIPAM-AA) for 40 min at room temperature. After activating the P(NIPAM-AA), 42 μl of 0.1 mM aptamer was added and reacted for 30 min at room temperature, followed by refrigeration at 4 °C overnight, in order to obtain aptamer-functionalized P(NIPAM-AA) through the formation of amide bonds.
The TiO2/P(NIPAM-AA) 1DPCs were fabricated at 2000 rpm in turn by the spin-coating method, both of which were spin-coated onto a silicon wafer, and different thicknesses were obtained by changing the time of spin-coating, the spin-coating rate, and the concentration of the solutions. Then, each layer was roasted at 37 °C for 35 min. In the experiments, eight layers were made in all and every period included two layers in that the first was P(NIPAM-AA) layer, and the last layer was titania layer. After four periods, the color of the TiO2/P(NIPAM-AA) 1DPCs was green, then the color appeared to be blue and the thickness decreased at the same time when dipping in the Hg2+ solution for 30 min (Scheme 1).
 | ||
| Scheme 1 Schematic illustration of the processes used to fabricate 1DPCs by spin-coating and structural illustration of TiO2/P(NIPAM-AA) 1DPCs for the detection of Hg2+. | ||
Measurement of reflective spectra
The spectra of the 1DPCs at normal incidence were recorded by using a microscope (Olympus BX51) equipped with a fiber optic spectrometer (Ocean Optics, QE65000) at an incidence angle of 0°.Measurements of scanning electronic microscopy (SEM)
The morphology images of the photonic crystal film were obtained with a Zeiss Ultra Plus field emission SEM which operated at 5 kV (Zeiss, Oberkochen, Germany).Measurement of FT-IR spectra
FT-IR spectra of the prepared samples were obtained using a Nicolet 5700 (Thermo Electron Scientific Instruments Corp).Results and discussion
Characterization of 1DPCs
Fig. 1 shows the reflective spectra of three TiO2/P(NIPAM-AA) 1DPCs, which were adjusted by controlling the time of spin-coating. The speed of spin-coating was 6000 rpm, and the periods of spin-coating are 3, 4, and 5 respectively. As the photonic stopbands fell in the visible region, the 1PDCs presented an obvious color. From the optical photographs in Fig. 1, we can see that the material with the photonic stopband at 478 nm presents a blue color, that at 550 nm presents a green color, and that at 620 nm presents a red color. It can be seen that the stopbands of TiO2/P(NIPAM-AA) 1PDCs will be tuned to full-colour range. | ||
| Fig. 1 Reflective spectra of three aptamer-functionalized 1DPCs with different periods. Insets: corresponding photographs. | ||
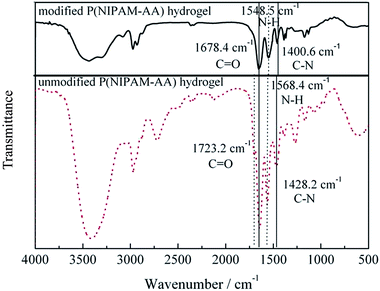
Fig. 2 shows the ATR-FT-IR spectra of unmodified and modified P(NIPAM-AA) hydrogels respectively. The major spectral peaks observed in the 1800–1400 cm−1 range are presented in Fig. 2. As shown in Fig. 2, for unmodified P(NIPAM-AA), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1723.2 cm−1, –NH at 1568.4 cm−1 and C–N at 1428.2 cm−1 all emerged as peaks corresponding to amido bond, which meant that P(NIPAM-AA) contained amide groups. In Fig. 2 for modified P(NIPAM-AA), C
O at 1723.2 cm−1, –NH at 1568.4 cm−1 and C–N at 1428.2 cm−1 all emerged as peaks corresponding to amido bond, which meant that P(NIPAM-AA) contained amide groups. In Fig. 2 for modified P(NIPAM-AA), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1723.2 cm−1 deviated to lower wavenumber and C
O at 1723.2 cm−1 deviated to lower wavenumber and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appeared at 1678.4 cm−1 due to the P–π conjugation between N atom lone electron pair and carbonyl. At the same time, the N–H peak at 1548.5 cm−1 deviated relative to the one at 1568.4 cm−1, which meant new amido bond appeared among them. Besides, due to the overlap of asymmetric COO− bands and amide II bands (NH bend and CN stretch of backbone amides), C–N at 1428.2 cm−1 becomes C–N at 1400.6 cm−1. The explanation for why the peak at 1400.6 cm−1 becomes narrow and sharp was the effects coming from the environment. In conclusion, all results indicate that aptamers were successfully immobilized in P(NIPAM-AA) hydrogel to prepare for making the TiO2/P(NIPAM-AA) 1DPCs.
O appeared at 1678.4 cm−1 due to the P–π conjugation between N atom lone electron pair and carbonyl. At the same time, the N–H peak at 1548.5 cm−1 deviated relative to the one at 1568.4 cm−1, which meant new amido bond appeared among them. Besides, due to the overlap of asymmetric COO− bands and amide II bands (NH bend and CN stretch of backbone amides), C–N at 1428.2 cm−1 becomes C–N at 1400.6 cm−1. The explanation for why the peak at 1400.6 cm−1 becomes narrow and sharp was the effects coming from the environment. In conclusion, all results indicate that aptamers were successfully immobilized in P(NIPAM-AA) hydrogel to prepare for making the TiO2/P(NIPAM-AA) 1DPCs.
 | ||
| Fig. 2 FT-IR spectra of unmodified P(NIPAM-AA) hydrogel (----) and modified P(NIPAM-AA) hydrogel (—). | ||
Fig. 3 shows the SEM image of a TiO2/P(NIPAM-AA) 1DPC modified by the aptamer (2N, N = 4, N is the number of TiO2 layers), which was fully prepared in the light of our current work, where we can see the evident multilayered construction. TiO2/P(NIPAM-AA) 1DPCs have four layers; that is to say, they include eight layers.
Principle of the 1DPCs for heavy metal Hg2+ detection
Scheme 2 shows the working principle of the 1DPCs for heavy metal Hg2+ detection. The aptamers in a hydrogel network captured the Hg2+ through a cross-linking mechanism, whose sequence was TTTCTTCTTTCTTCCCCCCTTGTTTGTTGTT-T.46,47 When the aptamers bound selectively with the Hg2+, they could adopt a random coil structure to form a construction of T–Hg2+–T complex (Scheme 2a). Because the structural changes of the aptamers were not generally watched, in order to solve the problem, we applied a novel aptamer-based sensor to obtain the visual and simple detection of Hg2+ by modifying the aptamer recognition elements with a TiO2/P(NIPAM-AA) 1DPCs film for the first time (Scheme 2b). As shown in Scheme 2b, 3′- and 5′-amino-modified aptamer was chemically coupled to the hydrogel network of this 1DPC in an EDC/NHS solution at first, followed by refrigeration at 4 °C after reacting at room temperature for 30 min. Then the aptamer conformation triggers shrinkage of this hydrogel which changed when the aptamer specifically bound with Hg2+. In the end, we measured the thickness of this TiO2/P(NIPAM-AA) 1DPCs film respectively in the Hg2+ solution or not in it. From Fig. 4, we can see that the thickness of the 1DPC specially bound with Hg2+ is about 190.4 nm (Fig. 4b). Comparing with this result, the thickness of the uncombined 1DPC is some 290.2 nm (Fig. 4a), so the thickness of the first one is less by 98.8 nm, whose period is three (six layers). This shows that the colored 1DPC film can report the process as a blue shift of its structure and the result is in accordance with the shrinkage of the hydrogel as a structure of T–Hg2+–T complex when aptamer is selectively combined with Hg2+. From the above, the color of this 1DPC film can be applied to qualitatively estimate Hg2+ ions, when the shift value of the diffraction peak is able to give a quantitative result for Hg2+. | ||
| Scheme 2 (a) The aptamer sequence for the detection of Hg2+. (b) Crosslinking reaction of aptamer with P(NIPAM-AA) and an illustration of hydrogel aptamer for the detection of Hg2+. | ||
 | ||
| Fig. 4 The SEM images of the TiO2/P(NIPAM-AA) 1DPC films. (a) Thickness of the 1DPCs in the absence of Hg2+. (b) Thickness of the 1DPCs in the presence of Hg2+. | ||
The quantitative behavior of the colorimetric analysis was evaluated with different concentrations of Hg2+ in pH 7.5 PBS buffer for 30 min.46 In the first place, this film displayed a yellow green color and a reflection peak at 575 nm. The film showed a notable structural color change and diffraction wavelength blue shift when the aptamer in the P(NIPAM-AA) hydrogel was bound with Hg2+. Besides, the color changes could be used for detecting Hg2+ relying on a calibration color chart as they are obvious and bright (shown in Fig. 5). As shown in Fig. 5, the peak shift value of the film reduced with an increase in the Hg2+ concentration from 0 nM to 5 μM and saturated at 5 μM Hg2+. That sensor maybe has some use because the toxic level of Hg2+ in drinking water is below 10 nM according to the US Environmental Protection Agency (US EPA).
 | ||
| Fig. 5 Photographs and reflection spectra of the colorimetric 1DPC films at different concentrations of Hg2+. Insets: corresponding photographs. | ||
Fig. 6 shows that the shift value increased with corresponding increasing Hg2+ concentration from 0 nM to 50 nM in Fig. 5. This proved that Hg2+ concentration changes would result in a peak shift, but there is not a linear correlation between Hg2+ concentration and peak shift. In our research, the 1DPC film was provided with selectivity to Hg2+ and was evaluated by testing the response of it to other environmentally relevant metal ions, such as Pb2+, Fe3+, Cu2+, Ag+ at a concentration of 100 μM, because Hg2+ commonly coexists with other heavy metals in the environment. A solution of only Hg2+ (5 μM) could induce a meaningful diffraction shift of the film; in sharp contrast, the other metal ions hardly had any effect (Fig. 7). Therefore, the 1DPC film exhibits good selectivity to Hg2+ against other associated metal ions and implies the potential of its use for actual samples.
 | ||
| Fig. 6 Diffraction blue shift of the film influenced by Hg2+ concentration. The solid line is a guide for the points. | ||
 | ||
| Fig. 7 The effect of various metal ions on the 1DPC sensor diffraction shift (5 μM for Hg2+ and 100 μM for Pb2+, Fe3+, Cu2+ and Ag+). | ||
Fig. 8 illustrates five cycles of the diffraction wavelength of the aptasensor with little change in the stopband. The concentration of mercury ions was 0 nM and 50 nM, respectively. The aptasensors were soaked in 1 ml of 1% HCl for 1 min. Then, they were washed three times with water and twice with reaction buffer. This process was repeated three times. After that, the sensors were used for Hg2+ detection again. In our experiment, we found that this Hg2+-responsive behavior could only be repeated five times. It is suspected that TiO2 could be dissolved because of being immersed in the alkaline or acid solution for a long time.50,51 The amorphous TiO2 reacts with alkalis or acids relatively easily compared with rutile titanium dioxide, anatase titanium dioxide, and titania nanoparticles. When the number of repeat times increases, the structure of the sensor may be damaged. We can use titania nanoparticle solution to replace TiO2 sol to solve this problem. It will be more stable if the crystal form of TiO2 converts to rutile and anatase.
Conclusions
In conclusion, a new type of TiO2/P(NIPAM-AA) 1DPCs colorimetric sensor film is made by the spin-coating technique. The 1DPCs have an obvious multilayered structure as determined through SEM images. The film including an Hg2+-responsive aptamer hydrogel can report the Hg2+ concentration as a prominent structural color variation and diffraction wavelength shift to qualitatively detect the Hg2+ concentration. It turns out that not only can the film detect a wide range of Hg2+ concentrations with selectivity and sensitivity but also its detection limit achieves the sensitivity needs in water of the US EPA. So the research supplies a simple and visual detection method for aptamer-based heavy metal Hg2+ ion analysis. We hope the technique could be widely applied in various fields, such as medical diagnostics, environmental monitoring, forensic analysis and so on.Acknowledgements
The authors are grateful to the Southeast University for providing space, apparatus, instrumentation, literature and financial support throughout the research experiment. Prof. Dr Ge is grateful to Prof. Dr Gu Zhongze's group for their support.Notes and references
- S. Y. Al-Mohanna and M. N. V. Subrahmanyam, Flux of heavy metal accumulation in various organs of the intertidal marine blue crab, Portunus pelagicus (L.) from the Kuwait coast after the Gulf War, Environ. Sci., 2001, 27, 321–326 CAS.
- A. R. Norris, I. Onyido and E. Buncel, Biomolecule–mercury interactions: modalities of DNA base-mercury binding mechanisms, remediation strategies, Chem. Rev., 2004, 104, 5911–5929 CrossRef PubMed.
- A. H. Environ, A review of the studies of the cardiovascular health effects of methylmercury with consideration of their suitability for risk assessment, Environ. Res., 2005, 98, 133–142 CrossRef PubMed.
- L. M. Campbell and D. G. Dixon, A review of mercury in Lake Victoria, East Africa: implications for human and ecosystem health, J. Toxicol. Environ. Health, Part B, 2003, 6, 325–356 CAS.
- F. M. M. Morel, A. M. L. Kraepiel and M. Amyot, The chemical cycle and bioaccumulation of mercury, Annu. Rev. Ecol. Syst., 1998, 29, 543–566 CrossRef.
- A. Dhamodaran, S. Arindam and J. Tushar, Photonic crystal hydrogel material for the sensing of toxic mercury ions (Hg2+) in water, Soft Matter, 2011, 7, 2592–2599 RSC.
- M. Morita, J. Yoshinaga and J. S. Edmonds, The determination of mercury species in environmental and biological samples (technical report), Pure Appl. Chem., 1998, 70, 1585–1615 CrossRef CAS.
- I. Pickering, H. H. Harris and G. N. Geofge, Tetrathiomolybdate causes formation of hepatic copper–molybdenum clusters in an animal model of Wilson's disease, Science, 2003, 301, 1704–1705 Search PubMed.
- F. Zino, A. Renzoni and E. Franchi, Mercury levels along the food chain and risk for exposed populations, Environ. Res., 1998, 77, 68–72 CrossRef PubMed.
- D. W. Boening, Ecological effects, transport, and fate of mercury: a general review, Chemosphere, 2000, 40, 1335–1351 CrossRef CAS PubMed.
- T. A. Baughman, Elemental mercury spills, Environ. Health Perspect., 2006, 114, 147–452 CrossRef PubMed.
- C. W. Liu, C. K. Chiang and C. C. Huang, Oligonucleotide-based fluorescence probe for sensitive and selective detection of mercury(II) in aqueous solution, Anal. Chem., 2008, 80, 3716–3721 CrossRef PubMed.
- O. T. Butler, J. M. Cook and C. F. Harrington, et al., Atomic spectrometry update, environmental analysis, J. Anal. At. Spectrom., 2006, 21, 217–245 RSC.
- R. G. Wuilloud, J. C. A. DeWuilloud and M. F. Silva, Sensitive determination of mercury in tap water by cloud point extraction pre-concentration and flow injection-cold vapor-inductively coupled plasma optical emission spectrometry, Spectrochim. Acta, Part B, 2002, 57, 365–374 CrossRef.
- B. Li, J. Sun and Y. X. Gao, Elimination efficiency of different reagents for the memory effect of mercury using ICP-MS, J. Anal. At. Spectrom., 2006, 21, 94–96 RSC.
- X. Li, S. Arzhantsev and J. F. Kauffman, Rapid Limit Tests for Metal Impurities in Pharmaceutical Materials by X-ray Fluorescence Spectroscopy Using Wavelet Transform Filtering, Anal. Chem., 2011, 83, 1061–1068 CrossRef PubMed.
- X. H. Qian, X. F. Guo and L. H. Jia, A highly selective and sensitive fluorescent chemosensor for Hg2+ in neutral buffer aqueous solution, J. Am. Chem. Soc., 2004, 126, 2272–2273 CrossRef PubMed.
- Y. Q. Huang, L. M. Zhang and H. Jiang, A cell compatible fluorescent chemosensor for Hg2+ based on a novel rhodamine derivative that works as a molecular keypad lock, RSC Adv., 2011, 1, 1294–1300 RSC.
- S. Mishra, M. Suresh and A. Das, The detection of Hg2+ by cyanobacteria in aqueous media, Chem. Commun., 2009, 2496–2498 Search PubMed.
- B. Kuswandi and W. Verboom, Selective chemosensor for Hg(II) ions based on tris[2-(4-phenyldiazenyl)phenylaminoethoxy]cyclotriveratrylene in aqueous samples, Anal. Chim. Acta, 2009, 655, 75–79 CrossRef PubMed.
- S. Dzyadevych, C. Durrieu and J. M. Chovelon, A bi-enzymatic whole cell conductometric biosensor for heavy metal ions and pesticides detection in water samples, Biosens. Bioelectron., 2005, 21, 273–281 CrossRef PubMed.
- L. Deng, S. J. Dong and S. J. Guo, Selective Decomposition of Formic Acid over Immobilized Catalysts, Anal. Chem., 2011, 83, 3968–3972 CrossRef PubMed.
- L. H. Hu, A. B. Iliuk and W. A. Tao, Identification of Drug Targets In Vitro and in Living Cells by Soluble-Nanopolymer-Based Proteomics, Anal. Chem., 2011, 83, 4133–4136 Search PubMed.
- J. Liu, Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors, Soft Matter, 2011, 7, 6757–6767 RSC.
- S. Huijie, H. Liu and Z. Zhu, A novel photoelectrochemical sensor based on molecularly imprinted polymer modified TiO2 nanotubes and its highly selective detection of 2,4-dichlorophenoxyacetic acid, Electrochem. Commun., 2010, 49, 1404–1407 Search PubMed.
- H. Togashi and A. Ono, Highly selective oligonucleotide-based sensor for mercury(II) in aqueous solutions, Angew. Chem., Int. Ed., 2004, 43, 4300–4302 CrossRef PubMed.
- Y. Y. Su, D. Li and S. P. Song, Highly Sensitive Electrochemical Sensor for Mercury(II) Ions by Using a Mercury-Specific Oligonucleotide Probe and Gold Nanoparticle-Based Amplification, Anal. Chem., 2009, 81, 7660–7666 CrossRef PubMed.
- M. Y. Chan, B. D. Smith and J. W. Liu, Regenerable DNA-Functionalized Hydrogels for Ultrasensitive, Instrument-Free Mercury(II) Detection and Removal in Water, J. Am. Chem. Soc., 2010, 132, 12668–12673 CrossRef PubMed.
- S. Y. Lim, L. Piao and T. D. Chung, Mercury(II) detection by SERS based on a single gold microshell, Chem. Commun., 2010, 46, 5587–5589 RSC.
- C. Li and C. Dong, Au NPs-enhanced surface plasmon resonance for sensitive detection of mercury(II) ions, Biosens. Bioelectron., 2010, 25, 2622–2626 CrossRef PubMed.
- B. C. Ye and B. C. Yin, Highly Sensitive Detection of Mercury(II) Ions by Fluorescence Polarization Enhanced by Gold Nanoparticles, Angew. Chem., Int. Ed., 2008, 47, 8386–8389 CrossRef CAS PubMed.
- E. Yablonovitch, Inhibited spontaneous emission in solidstate physics and electronics, Phys. Rev. Lett., 1987, 58, 2059–2062 CrossRef CAS PubMed.
- S. John, Strong localization of photons in certain disordered dielectric superlattices, Phys. Rev. Lett., 1987, 58, 2486–2489 CrossRef CAS PubMed.
- J. M. Weissman, H. B. Sunkara, A. S. Tse and S. A. Asher, Thermally switchable periodicities and diffraction from mesoscopically ordered materials, Science, 1996, 274, 959–960 CrossRef CAS PubMed.
- S. A. Asher and J. H. Holtz, Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials, Nature, 1997, 389, 829–832 CrossRef PubMed.
- Y. Bingwu and Z. Liping, Application of Nanometer Zirconium Oxide Immobilized on Silica Gel as a Solid Phase Extraction Sorbent for the Preconcentration of Pb in Water Samples Prior to Flame Atomic Absorption Spectrometry Determination, At. Spectrosc., 2011, 32, 1264–1270 Search PubMed.
- S. Pal, A. R. Yadav, M. A. Lifson, J. E. Baker, P. M. Fauchet and L. Miller, Selective virus detection in complex sample matrices with photonic crystal optical cavities, Biosens. Bioelectron., 2013, 44, 229–234 CrossRef CAS PubMed.
- W. Z. Shen, M. Z. Li, L. Xu, S. T. Wang, L. Jiang, Y. L. Song, S. D. Wang and B. Zhu, Highly effective protein detection for avidin-biotin system based on colloidal photonic crystals enhanced fluoroimmunoassay, Biosens. Bioelectron., 2011, 26, 2165–2170 CrossRef CAS PubMed.
- V. Morandi, F. Marabelli and V. Amendola, Colloidal photonic crystals doped with gold nanoparticles: spectroscopy and optical switching properties, Adv. Funct. Mater., 2007, 17, 2779–2786 CrossRef CAS.
- L. D. Bonifacio, B. V. Lotsch and D. P. Puzzo, Stacking the Nanochemistry Deck: Structural and Compositional Diversity in One-Dimensional Photonic Crystals, Adv. Mater., 2009, 21, 1641–1646 CrossRef CAS.
- L. T. Zhang, W. F. Xie, J. Wang, H. Z. Zhang and Y. S. Zhang, Optical properties of a periodic one-dimensional metallic-organic photonic crystal, J. Phys. D: Appl. Phys., 2006, 39, 2373–2376 CrossRef CAS.
- R. M. Almeida and A. S. Rodrigues, Photonic bandgap materials and structures by sol–gel processing, J. Non-Cryst. Solids, 2003, 326, 405–409 CrossRef.
- Y. Fink, J. N. Winn, S. H. Fan, C. P. Chen, J. Michel, J. D. Joannopoulos and E. L. Thomas, A dielectric omnidirectional reflector, Science, 1998, 282, 1679–1682 CrossRef CAS PubMed.
- V. K. S. Hsiao, W. D. Kirkey and F. Chen, Organic solvent vapor detection using holographic photopolymer reflection gratings, Adv. Mater., 2005, 17, 2211–2214 CrossRef CAS.
- V. Morandi, F. Marabelli, V. Amendola, M. Meneghetti and D. Comoretto, Colloidal photonic crystals doped with gold nanoparticles: spectroscopy and optical switching properties, Adv. Funct. Mater., 2007, 17, 2779–2786 CrossRef CAS.
- B. F. Ye, Y. J. Zhao, Y. Cheng, T. T. Li, Z. Y. Xie, X. W. Zhao and Z. Z. Gu, Colorimetric photonic hydrogel aptasensor for the screening of heavy metal ions, Nanoscale, 2012, 4, 5998–6003 RSC.
- Y. Miyake, H. Togashi, M. Tashiro, H. Yamaguchi, S. Oda, M. Kudo, Y. Tanaka, Y. Kondo, R. Sawa, T. Fujimoto, T. Machinami and A. Ono, J. Am. Chem. Soc., 2006, 128, 2172–2173 CrossRef CAS PubMed.
- G. Wang, J. Wang, S. Y. Li, J. W. Zhang and C. W. Wang, One-dimensional alumina photonic crystals with a narrow band gap and their applications to high-sensitivity concentration sensor and photoluminescence enhancement, Superlattices Microstruct., 2015, 86, 546–551 CrossRef CAS.
- I. R. Howell, C. Li, N. S. Colella, K. Ito and J. J. Watkins, Strain-tunable one dimensional photonic crystals based on zirconium dioxide/slide-ring elastomer nanocomposites for mechanochromic sensing, ACS Appl. Mater. Interfaces, 2015, 7, 3641–3646 CAS.
- J. H. Zhang, Z. H. Wang, Z. C. Tian, Z. Y. Wang, Y. F. Li, H. Zhang, S. Liang and B. Yang, Organic/inorganic hybrid photonic hydrogels as a colorful platform for visual detection of SCN−, Chem. Commun., 2010, 46, 8636–8638 RSC.
- C. C. Tsai and H. S. Teng, Structural features of nanotubes synthesized from NaOH treatment on TiO2 with different post treatments, Chem. Mater., 2006, 18, 367–373 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |


