Open Access Article
Open Access ArticlePhosphorescent oxygen-sensing and singlet oxygen production by a biosynthetic silk†
Conor C. Horganab,
Yong-Shen Hanc,
Holly Truemanb,
Colin J. Jacksonc,
Tara D. Sutherlandb and
Trevor D. Rapson*b
aResearch School of Engineering, The Australian National University, Acton, ACT 2601, Australia
bCSIRO, Black Mountain, Acton, ACT 2601, Australia. E-mail: trevor.rapson@csiro.au
cResearch School of Chemistry, The Australian National University, Acton, ACT 2601, Australia
First published on 12th April 2016
Abstract
A recombinant coiled-coil silk was utilised to immobilise heavy-metal-macrocycles which are known to undergo efficient intersystem crossing from the singlet state to the triplet state following excitation with visible light. This spin-forbidden transition leads to phosphorescence and the production of cytotoxic oxygen species. We explore the requirements for specific binding of these macrocycles and demonstrate that immobilisation does not adversely affect their photochemical properties. The biocompatible materials developed here have potential biomedical applications in photodynamic therapy (PDT) and dynamic oxygen-sensing.
Recombinant coiled-coil silk proteins from organisms such as the honeybee Apis mellifera are potentially useful biomaterials owing to several features: they can be sterilised using methods such as gamma irradiation, they can be biodegraded into amino acids through the action of proteases,1 and they can be formed into a range of biocompatible solid-state materials such as films, sponges and fibres that are stable at a wide range of temperatures.2–4
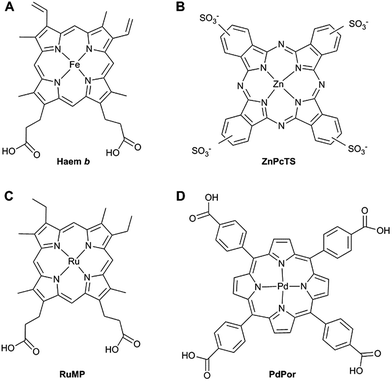
We have recently reported that haem cofactors (Fig. 1A) can be introduced into materials generated from recombinant honeybee silk proteins. Of particular interest was the fact that the silk protein coordinated directly to the iron centre in a manner reminiscent of naturally occurring haem proteins,5 one of the principle ways the reactivity of the metal centre is controlled in these proteins.6 We demonstrated that the haem-silk materials have nitric oxide-sensing properties and remained functional when stored at room temperature as dry films for over one year.5
The photochemical and photophysical properties of porphyrin-based macrocycles make them useful for a range of biomedical applications such as photodynamic therapy (PDT).7,8 PDT uses a photosensitiser, activated by the absorption of visible light, to form reactive oxygen species such as singlet oxygen which are cytotoxic to cells such as cancerous tumours.
With the rise of multi-drug resistance, there is renewed interest in photodynamic antimicrobial chemotherapy (PACT).8 Given the mode of action of PACT, using reactive oxygen species, it is virtually impossible for resistant strains to evolve.9–11
Traditionally PDT and PACT rely on photosensitiser accumulation thereby producing the cytotoxic species within the cell of interest.8 However, it has been demonstrated that cell accumulation is not necessary for antimicrobial activity and pure singlet oxygen, produced externally, is cytotoxic to bacteria.12 This cytotoxic property of singlet oxygen allows immobilisation of photosensitisers in materials such as cellulose films13 and paper,14 cotton fabric15 and nylon fibres16 while maintaining cytotoxic activity. Using a biocompatible material such as recombinant silk could be advantageous for a number of applications including water treatment plants and antimicrobial wound dressings.10
In addition to PDT, porphyrinic compounds have also been investigated for use in oxygen sensors. This optical method uses the quenching of photoluminescence by molecular oxygen,17 with the rate of phosphorescence decay being inversely proportional to the concentration of oxygen quenching molecules.
Phosphorescent oxygen-sensing overcomes the disadvantage of traditional Clark electrodes which require the consumption of oxygen and provides a non-invasive means by which to measure oxygen concentrations in vivo and in vitro.18–20
Here we sought to extend the functional properties of biocompatible coiled-coil silk proteins by replacing the haem cofactor with bio-inspired synthetic macrocycles that are known to efficiently produce singlet oxygen or that are suitable for dynamic oxygen sensing.
We previously proposed that the negative charge of the propionate groups on haem b was important for the binding of the porphyrin ring to the silk, given that the recombinant silk is rich in positively charged amino acids such as lysine.5 Therefore, we sought to find synthetic porphyrin macrocycles with a similar negative charge.
Zinc phthalocyanine tetrasulfonate (ZnPcTs) is one such macrocycle that meets these requirements (Fig. 1B). This compound has been widely used as a photosensitiser in PDT and is an efficient producer of singlet oxygen.21,22 We set out to determine if ZnPcTs will bind to recombinant silk materials and whether binding of the phthalocyanine to the silk affects its ability to produce singlet oxygen.
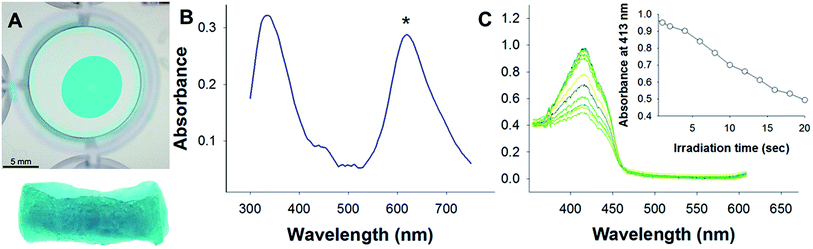
ZnPcTs readily binds to honeybee silk materials by soaking a preformed film or sponge in a 70% methanol solution with ∼1 mg mL−1 of ZnPc tetrasulfonate (Experimental details in ESI†) to produce transparent blue silk materials with absorbance maxima at 335 and 620 nm (Fig. 2A and B). Zinc phthalocyanine, which lacks the sulfonate groups and hence the negative charges of ZnPcTs, did not detectably bind to the silk materials, leaving the films and sponges colourless (ESI Fig. S1†). This demonstrates the importance of the negatively charged moieties decorating the macrocycles for binding to the silk protein.
The production of singlet oxygen was tested using a standard procedure outlined by Kochevar and Redmond, whereby singlet oxygen bleaches the absorbance of a 1,3-diphenylisobenzofuran (DPRF).23 When silk films with ZnPcTs were irradiated using a tungsten bulb with a red pass filter, a pronounced decrease in the absorbance of DPRF at 413 nm was noted (Fig. 2B). To confirm that the degradation was due to the production of singlet oxygen and not photobleaching, oxygen was removed from the DPRF solutions by thorough purging with argon gas. The rate of DPRF degradation was significantly reduced following purging with argon (ESI Fig. S2†).
In the experiments described above, DMSO was used as the solvent, as DPRF is not soluble in aqueous solutions. The silk materials were found to be stable in DMSO and the immobilised ZnPcTs remained bound to the film. This compatibility with DMSO is similar to what we have noted previously for solid-state silk materials, which are stable in solvents such as chloroform and acetone.5 We note that this solvent stability means that these singlet oxygen producing materials could be used for organic polymerization reactions driven by singlet oxygen.24
Having established that phthalocyanines incorporated into silk films are able to produce singlet oxygen, we shifted our attention to optical oxygen sensing macrocycles. We25,26 and others20,27,28 have used metal-ion substituted porphyrins to introduce new chemical properties to haem proteins. First, we selected to use a ruthenium metal centre as it has been reported to promote the spin-forbidden transitions necessary for phosphorescence but retain similar axial coordination to iron.20,29 In addition, we employed a mesoporphyrin (RuMP) rather than a protoporphyrin given the improved photostability of mesoporphyrins, which lack the reactive vinyl groups (Fig. 1A vs. C).30 Secondly, palladium-meso-tetra-(4-carboxyphenyl) porphyrin (PdPor – Fig. 1D) was tested for its compatibility with the silk proteins, as PdPor is the chromophore of the commercially available phosphor, oxyphor R2. In oxyphor R2, PdPor is bound to second generation dendrimers to improve their solubility in water and physiological fluids.31 Recently, oxyphor R2 has been used to monitor cutaneous oxygenation by incorporating the porphyrin–dendrimer into rapid-drying liquid bandages.32
Both RuMP and PdPor were found to bind to honeybee silk materials from a 70% methanol solution (Fig. 3A and B). The binding of PdPor, however, was observed to be weaker than that of RuMP on the basis that PdPor could be removed by washes with 70% methanol. The binding of PdPor to the silk was, however, strong in biologically relevant buffers such as phosphate buffered saline. The weaker binding of the PdPor to the silk films could be a result of increased steric bulk from the carboxyphenyl substituents compared to RuMP.
When both PdPor and RuMP were immobilised in the silk films they showed oxygen-sensitive emission peaks at 660 nm and 690 nm (Fig. 3C and D). These emission peaks can be attributed to the phosphorescence of the immobilised macrocycles. The position of the emission peaks, at 660 nm and 700 nm for RuMP and PdPor in silk films respectively, is similar to that observed for the macrocycle in 70% methanol and that reported previously in aqueous buffers.20,31
To determine the effects of immobilisation in silk on the macrocycles, the spectroscopic and photochemical properties of the modified silks were compared to the macrocycles alone. The most pronounced change apparent after incorporation into the film was a dramatic increase in the phosphorescent lifetime of the phosphors (Table 1). The increased phosphorescent lifetimes are similar to that reported for oxyphor R2 (∼700 μs at room temperature) compared to unmodified PdPor.31 Extended phosphorescent lifetimes such as these are advantageous as they allow autofluorescence to be illuminated from biological samples using time-gated measurements.33
| Lifetime | In solution | In silk film | ||||
|---|---|---|---|---|---|---|
| +O2 (μs) | −O2 (μs) | τ0/τ | +O2 (μs) | −O2 (μs) | τ0/τ | |
| Ruthenium mesoporphryin | ||||||
| τ1 | 0.199 | 0.283 | 1.42 | 18.7 | 19.9 | 1.06 |
| τ2 | 0.994 | 1.27 | 1.28 | 59.2 | 65.0 | 1.10 |
| τ3 | 10.9 | 12.5 | 1.15 | 176 | 199 | 1.13 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Palladium-meso-tetra-(4-carboxyphenyl)porphine | ||||||
| τ1 | 0.927 | 2.44 | 2.63 | 413 | 483 | 1.17 |
| τ2 | 9.948 | 19.6 | 1.98 | 809 | 836 | 1.03 |
Although both RuMP and PdPor showed oxygen-sensitive emission peaks, which were unaffected by immobilisation in a silk film, there were pronounced changes in the non-oxygen-sensitive emission peaks. This was particularly the case for RuMP (ESI Fig. S3†). When immobilised in silk, the emission peak at 620 nm (in solution) blue shifted to 480 nm (in film). The fact that this peak is oxygen-insensitive, suggests the emission is due to fluorescence rather than phosphorescence. The change in emission wavelength noted with incorporation into silk materials is most likely due to coordination of an amino acid residue to the ruthenium centre, similar to what has previously been noted for haem b.5
The immobilisation of the macrocycles within the silk films also improved the photostability of the macrocycles (ESI Fig. 4†). The improvement was most prominent for PdPor for which absorbance at 530 nm showed a minor decrease in intensity over 12 days compared to PdPor in 70% methanol that showed a >50% decrease over 5 days. This effect has been previously observed for PdPor bound to albumin, which was suggested to result from restricted oxygen access to the phosphor, thereby reducing the rate that reactive oxygen species were produced.19 These results highlight the stabilising and protecting role the silk film plays for PdPor.
One of the main advantages of using biomaterials generated from recombinant silk proteins is that they can be stored as a dry film while retaining their functional properties.4,5 We have previously reported the use of myoglobin-modified silk films to detect nitric oxide directly from a gaseous sample, rather than requiring the gas to be dissolved in a solution.4 Given this unique property, our observation that the modified silk films produced here were able to detect oxygen in gaseous samples as well as dissolved oxygen was notable. As the silk materials can be either hydrated or dried to become solvent-free, the macrocycle-modified silk films employed here could be used to detect oxygen both in solutions and from gaseous samples. This property broadens the sample range that can be analysed using immobilisation in silk films.
Through varying the macrocycle incorporated within biosynthetic silk films, we have been able to produce bioinorganic materials that can undergo the spin-forbidden singlet–triplet transition. This has allowed us to produce stable, biocompatible, materials that could be used for oxygen-sensing and antimicrobial activity.
Acknowledgements
We wish to thank Prof Elmars Krausz for the loan of a light source and filters to carry out singlet oxygen production experiments and Dr Helen Dacres for her assistance in carrying out fluorescent lifetime measurements.Notes and references
- C. R. Wittmer, X. Hu, P.-C. Gauthier, S. Weisman, D. L. Kaplan and T. D. Sutherland, Acta Biomater., 2011, 7, 3789–3795 CrossRef CAS PubMed.
- M. G. Huson, J. S. Church, J. M. Poole, S. Weisman, A. Sriskantha, A. C. Warden, P. M. Campbell, J. A. M. Ramshaw and T. D. Sutherland, PLoS One, 2012, 7, e52308 CAS.
- J. Poole, J. S. Church, A. L. Woodhead, M. G. Huson, A. Sriskantha, I. L. Kyratzis and T. D. Sutherland, Macromol. Biosci., 2013, 13, 1321–1326 CrossRef CAS PubMed.
- T. D. Rapson, J. S. Church, H. E. Trueman, H. Dacres, T. D. Sutherland and S. C. Trowell, Biosens. Bioelectron., 2014, 62, 214–220 CrossRef CAS PubMed.
- T. D. Rapson, T. D. Sutherland, J. S. Church, H. E. Trueman, H. Dacres and S. C. Trowell, ACS Biomater. Sci. Eng., 2015, 1, 1114–1120 CrossRef CAS.
- T. L. Poulos, J. Biol. Inorg. Chem., 1996, 1, 356–359 CrossRef CAS.
- M. G. Vicente, Curr. Med. Chem.: Anti-Cancer Agents, 2001, 1, 175–194 CrossRef CAS PubMed.
- H. Abrahamse and M. R. Hamblin, Biochem. J., 2016, 473, 347–364 CrossRef CAS PubMed.
- F. F. Sperandio, Y.-Y. Huang and M. R. Hamblin, Recent Pat. Anti-Infect. Drug Discovery, 2013, 8, 108–120 CrossRef CAS.
- C. Spagnul, L. C. Turner and R. W. Boyle, J. Photochem. Photobiol., B, 2015, 150, 11–30 CrossRef CAS PubMed.
- Y.-M. Jeon, H.-S. Lee, D. Jeong, H.-K. Oh, K.-H. Ra and M.-Y. Lee, Life Sci., 2015, 124, 56–63 CrossRef CAS PubMed.
- T. A. Dahl, R. W. Midden and P. E. Hartman, Photochem. Photobiol., 1987, 46, 345–352 CrossRef CAS PubMed.
- M. Krouit, R. Granet, P. Branland, B. Verneuil and P. Krausz, Bioorg. Med. Chem. Lett., 2006, 16, 1651–1655 CrossRef CAS PubMed.
- J.-P. Mbakidi, K. Herke, S. Alvès, V. Chaleix, R. Granet, P. Krausz, S. Leroy-Lhez, T.-S. Ouk and V. Sol, Carbohydr. Polym., 2013, 91, 333–338 CrossRef CAS PubMed.
- C. Ringot, V. Sol, R. Granet and P. Krausz, Mater. Lett., 2009, 63, 1889–1891 CrossRef CAS.
- J. Bozja, J. Sherrill, S. Michielsen and I. Stojiljkovic, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2297–2303 CrossRef CAS.
- S. M. Borisov and O. S. Wolfbeis, Chem. Rev., 2008, 108, 423–461 CrossRef CAS PubMed.
- R. Dedic, A. Svoboda, J. Psencik, L. Lupinkova, J. Komenda and J. Hala, J. Lumin., 2003, 103, 313–317 CrossRef.
- L. W. Lo, S. A. Vinogradov, C. J. Koch and D. F. Wilson, in Oxygen Transport to Tissue Mix, ed. D. K. Harrison and D. T. Delpy, Springer, New York, 1997, vol. 428, pp. 651–656 Search PubMed.
- M. B. Winter, E. J. McLaurin, S. Y. Reece, C. Olea, D. G. Nocera and M. a. Marletta, J. Am. Chem. Soc., 2010, 132, 5582–5583 CrossRef CAS PubMed.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19–33 RSC.
- J. W. Owens, R. Smith, R. Robinson and M. Robins, Inorg. Chim. Acta, 1998, 279, 226–231 CrossRef CAS.
- I. E. Kochevar and R. W. Redmond, in Methods in Enzymology, Elsevier, 2000, vol. 319, pp. 20–28 Search PubMed.
- S. Shanmugam, J. Xu and C. Boyer, J. Am. Chem. Soc., 2015, 137, 9174–9185 CrossRef CAS PubMed.
- T. D. Rapson, H. Dacres and S. C. Trowell, RSC Adv., 2014, 4, 10269–10272 RSC.
- T. D. Rapson, S. Warneke, M. M. Musameh, H. Dacres, B. C. T. Macdonald and S. C. Trowell, RSC Adv., 2015, 5, 89003–89008 RSC.
- M. B. Winter, P. J. Klemm, C. M. Phillips-Piro, K. N. Raymond and M. A. Marletta, Inorg. Chem., 2013, 52, 2277–2279 CrossRef CAS PubMed.
- P. C. Ford and I. M. Lorkovic, Chem. Rev., 2002, 102, 993–1018 CrossRef CAS PubMed.
- D. R. Paulson, A. W. Addison, D. Dolphin and B. R. James, J. Biol. Chem., 1979, 254, 7002–7006 CAS.
- K. M. Kadish, The Porphyrin Handbook: Synthesis and organic chemistry Vol 1, Elsevier, 2000, p. 33 Search PubMed.
- I. Dunphy, S. A. Vinogradov and D. F. Wilson, Anal. Biochem., 2002, 310, 191–198 CrossRef CAS PubMed.
- Z. Li, E. Roussakis, P. G. L. Koolen, A. M. S. Ibrahim, K. Kim, L. F. Rose, J. Wu, A. J. Nichols, Y. Baek, R. Birngruber, G. Apiou-Sbirlea, R. Matyal, T. Huang, R. Chan, S. J. Lin and C. L. Evans, Biomed. Opt. Express, 2014, 5, 3748–3764 CrossRef CAS PubMed.
- Q. Zhao, C. Huang and F. Li, Chem. Soc. Rev., 2011, 40, 2508–2524 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra03731c |
| This journal is © The Royal Society of Chemistry 2016 |