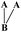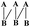A tough self-assembled natural oligomer hydrogel based on nano-size vesicle cohesion
Kai Li†
a,
Zhengdong Pan†a,
Cheng Guanb,
Hua Zhenga,
Kun Lia and
Hong Zhang*a
aResearch Institute of Resources Insects, Chinese Academy of Forestry, Kunming, 650224, People's Republic of China. E-mail: kmzhh@hotmail.com; Tel: +86-871-63860021
bFaculty of Material Engineering, Southwest Forestry University, Kunming, 650224, People's Republic of China
First published on 29th March 2016
Abstract
Shellac is an ancient oligomer and had been widely used in many fields in the past. Recently, we found that these low-molecular weight, biodegradable and amphiphilic biomacromolecules could aggregate to fabricate multi-scale materials. In this study, gluconic acid lactone (GDL) was employed as a H+ donor, and mixed with shellac-COONa to prepare a shellac hydrogel. It was found that in the beginning of gelation, as the hydrolysis of GDL proceeded, the amphiphilic molecules shellac-COONa changed to shellac-COOH and assembled into the nano-size vesicles. Furthermore, these vesicles constructed by shellac-COOH, similar to asymmetric gemini surfactants, could aggregate together to fabricate the network of the shellac hydrogel by the hydrophobic association between two sets of short hydrophobic chains from two adjacent vesicles. The shellac hydrogel has excellent mechanical properties and nice degradation behavior, and the compressive fracture stress, strain and compress modulus could be 7.6 MPa, 43.7%, 61 MPa, which means it can be used as a new kind of soft material, such as high mechanical performance bio-based foams.
Introduction
Shellac (Mw ≈ 1000), a kind of natural oligomer, is purified from the secretion of lac insects, which has excellent degradability and adhesivity, and could be used as a safe food additive approved by USA food and drug administration.1 Moreover, in recent years, shellac has been used in many different fields such as the waterproof sealing materials,2 medicine coatings,3 food surfactants,4,5 bioengineering materials6 and so on.As shown in Fig. 1,7 shellac consisted of aleuritic acid and cyclic terpene acid, both of which connected each other by the ester bond consuming the terminal carboxyl of aleuritic acid. The carboxyl of the cyclic terpene acid is the key group to control the hydrophilicity of the whole molecule.8 So, shellac could be used as a kind of amphiphilic polyelectrolyte, specifically, the aleuritic acid and cyclic terpene acid could be assumed as the hydrophobic segment and hydrophilic segment respectively.
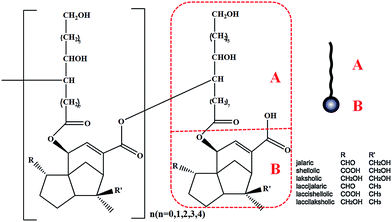 | ||
| Fig. 1 Generalised structure of shellac, with structures for aleuritic acid (A) and cyclic terpene acid (B).7 | ||
The aleuritic (9,10,16-trihydroxypalmitic) acid is a typical HFA molecule, that is also one segment of the natural resin shellac, as revealed – in Fig. 1. It is known that HFA is a kind of long chain alkyl hydroxyl carboxylic acid and is also the monomer unit of several natural biopolymers such as cutin.9 There is one terminal hydroxyl, one terminal carboxyl and some extra hydroxyl groups in the long chain alkyl molecule of HFA. Correspondingly, the long chain alkyl and terminal carboxyl of HFA act as the hydrophilic and hydrophobic segments of the surfactant like normal fatty acids that could induce hydrophobic interaction and cause the aggregation behavior when the pH is changed in aqueous phase.10 In addition, the abundant –OH groups of HFA could construct the H-bond intermolecular network, by which HFA could self-assemble into crystal with themselves or similar molecule on the mica11,12 or nano-scale micelles, multilamellar tubes in aqueous state.13–16 Therefore, the study of the HFA self-assembly behavior in aqueous is very amusing and has attracted much attention. Many kinds of functional materials could be constructed in aqueous phase, such as ultimate stable and temperature-responsive17–19 foam, photoresponsive foam14 and other nano-particles.20,21 The process of the HFA self-assembly in aqueous can be referred to the result of the cooperation of the hydrophobic interaction and hydrogen bonding. Briefly, at first, when the pH decreases, the hydrophobic interaction plays a dominant role in the aqueous phase that could induce the aggregation of the molecules, and shorten the distance between the molecules. Then, driven by the hydrogen bonding interaction between –OH groups, the HFA could self-assemble into solidified multi-size materials, which is totally different from the dynamic micelles made from the classic fatty acid or other surfactants. We assumed that if it could be used as the active segment in the macromolecule, like equipping the molecule with a “motor”, it could initiate self-assembly by the hydrophobic interaction and H-bond.
Shellac owns a special molecule structure with HFA segment which is “not only similar to simple surfactant, but also like asymmetric “Gemini” surfactant. The monomer of shellac has “Gemini” surfactant structure that can be abbreviated as “–C16–C8–” (Table 2). In 2003, Menger22 found that the asymmetric Gemini surfactants could self-assemble into vesicles, which in turn, self-assemble into gels, and attribute this phenomenon to the association of short hydrophobic chains from different vesicles. Based on the vesicles cohesion, many interesting materials were fabricated.23–25
| Time | Compressive properties | ||
|---|---|---|---|
| σ (MPa) | ε (%) | E (MPa) | |
| 6 | 0.77 | 56.2 | 5.1 |
| 12 | 2.21 | 49.6 | 19.6 |
| 18 | 2.74 | 49.8 | 21.2 |
| 24 | 3.64 | 45.4 | 28.5 |
| 36 | 7.67 | 44.2 | 61 |
In this case, the shellac was chosen as the object to study the self-assembly behavior of the hydroxy fatty acid with asymmetric gemini structure in aqueous phase resulting in the shellac hydrogel. We employed the GDL as the controlled-release H+ donor to change the pH of the system,26 and then to trigger the aggregation of the oligomer.
Experiment
Materials and chemicals
Shellac was purchased from Kunming Xilaike Bio-technology CO. Ltd (Kunming, Yunnan, China). Gluconic acid lactone and other organic reagents were purchased from Aladdin industrial Inc. Ltd (Shanghai, China). The water used in this study was ultrapure water.Preparation of shellac hydrogel
Firstly, the desired weight shellac was dissolved in the 1 mol L−1 Na2CO3 to fabricate the Shellac-COONa (the molar rate shellac/Na2CO3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the molecular weight was characterized by MALDI-TOF-MASS (Microflex, Bruker, Germany).26 Then, the acquired GDL was put into the solvent and mixed extensively for 5 min on a magnetic stirrer keeping at 25 °C for certain time. After the gelation finished, the “raw” shellac hydrogel was immersed into distilled water for one day to remove the residual product of GDL hydrolysis.
2), the molecular weight was characterized by MALDI-TOF-MASS (Microflex, Bruker, Germany).26 Then, the acquired GDL was put into the solvent and mixed extensively for 5 min on a magnetic stirrer keeping at 25 °C for certain time. After the gelation finished, the “raw” shellac hydrogel was immersed into distilled water for one day to remove the residual product of GDL hydrolysis.
Analysis of gel process of shellac hydrogel
The surface tension of aqueous solution of the shellac-Na was measured with a tensiometer by the Wilhelmy plate technique (K12, Krüss, Germany) at 25 °C. To investigate the shellac gel process, the equal molar dose of GDL was added in 8 wt% shellac-COONa solvent and kept at 25 °C for different time (6 h, 12 h, 18 h, 24 h, 36 h) to fabricate shellac hydrogels, meanwhile the variation of the volume, water content, pH of these hydrogels and the mixed solvent system were investigated. To observe the morphology changes of these hydrogels, the scanning electron microscopy (IGMA, Zeiss, Oberkochen, Germany) was used in this study. The Fourier transform infrared spectroscopy (FTIR) (Tenson 27, Bruker, Leipzig, Germany) was employed to investigate the mechanism of gelation process and interaction between GDL and shellac. The FTIR samples were prepared in the liquid sample cell that used CaF2 as the observation window. The mixture of GDL and shellac-COONa (3 wt%) with equal molar ratio was mixed in water for 5 min, and then put into the cell for observation, so did the GDL. The mechanical properties of the shellac hydrogels were characterized by compression tests, which were performed on a universal testing machine (CMT6350, Shenzhen, SANS, China) according to ISO527-3-1995 (E) at a speed of 5 mm min−1. The size of the hydrogel specimens for compression tests was 6 mm (diameter) × 15 mm (high). WAXD measurements were carried out on WAXD diffractometer (D8-Advance, Bruker, Karlsruhe, Germany) in reflection mode. The X-ray used was Ni-filtered Cu Kα radiation with a wavelength of 1.5418. The voltage was set at 40 kV and the current was set at 40 mA. Measurement was done by 2θ = 2° min−1 scan over 2θ range of 8 to 40°. The low concentration shellac-Na (0.01 wt%) aqueous solution was mixed with equal molar GDL to fabricate the shellac hydrogel dispersion, and then were examined by a transmission microscopy (TEM, JEM-2010, JEOL, Japan) to obtain topography and phase images in tapping mode. For the TEM, the samples were prepared by casting the shellac hydrogel solution onto a holey carbon film, which was supported on a copper grid. The degradation behavior of shellac hydrogel obtained over 36 h was measured as follows: specimens were placed in tubes filled with isoosmolar phosphate buffer (pH = 7.2). The tubes were placed in a water bath at 37 °C for certain periods of time. At each time point, the specimens were taken out, rinsed and then vacuum dried for analysis. The degradation was indicated by the following formula where m0 and mt are weight of the nanocomposites before and after degradation: loss weight ratio (%) = 100 × (m0 − mt)/m0.Results and discussion
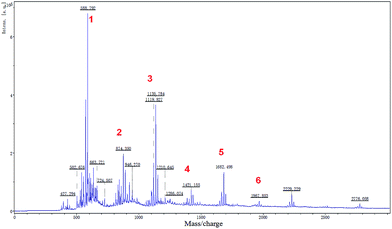
Shellac was not a familiar biomacromolecule to us. However, before petroleum based polymer, shellac was the common plastic in the market and used as water barrier in the military and food industry27,28 and had other applications in the electrical29 and medical3 industry, vinyl record being their “all-star” product in the past. Because of the higher price and the batch difference, shellac was taken off the mainstream market. Recently, with the rise of environmental consciousness, this ancient biomacromolecule is back and arouses much attention from scientists.3,6,30–32 In summary of recent works, more studies were found focusing on the shellac aggregation behavior.3–5,33–42 For example, the Velikov group3–5,34,43 have made many interesting works. They used shellac as a new surfactant of oleogels and prepared many kinds of shellac colloidal particle. Paunov38–41 also fabricated shellac composite microcapsules based on the shellac aggregation behavior of shellac in aqueous. After analysis of this research, we found that shellac could aggregate to assemble functional micro-materials. The driving force of this aggregation behavior may be attributed to the hydrophobic interaction or electrostatic interaction with other stuff. Meanwhile, the H-bond and van der Waals force may play important roles in the assembly process of micro-materials. But, there still exist some questions of why this low-molecular-weight biomacromolecule could assemble into multi-scale materials with continuous phase such as nano-scale43 particles, micro-scale particles41 and microfibers6 which were different from normal low-molecular-weight amphiphilic molecule? What was the key factor in the assembly process and whether shellac could assemble into more large-scale materials? No specific reference was given.Therefore, we employed the MALDI-TOF-MS method44 to determine the molecular distribution of shellac-Na. As Fig. 2 showed, to eliminate the effect of impurity, we only chose the peaks with molecule weight (MW) > 400. The details were listed in Table 1. It can indicate that shellac was a kind of alternative copolyesters which consist of aleuritic acids (part A) and sesquiterpene acids (part B) and it's worth noting that shellac had an asymmetric molecule structure, the lactone bond (C9) divided aleuritic acid into two parts (C7–C9).7,45 We found that this special structure was similar to the asymmetric gemini surfactants. In 2003, Menger found that the asymmetric gemini surfactants could self-assemble into vesicles which, in turn, self-assemble into gels.22 After Menger's discovery was published, many interesting materials were fabricated, especially the hydrogel.24,25,46
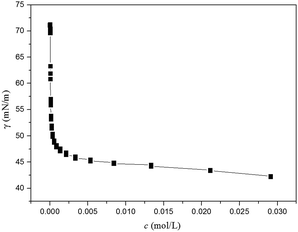
We also assumed that this special structure may be the key factor of aggregation behavior of shellac, and we also attempted to fabricate the shellac hydrogel. The surface tension curve of shellac-Na was showed in Fig. 3, an inflection point occurred apparently, which is similar to the critical micelle concentration of the classic surfactant. It was indicated that shellac-Na could display surface activity and aggregate above the certain contraction.
Analysis of gel process of shellac hydrogel
There were two steps in the gel formation of shellac as indicated in Fig. 4A, which included the hydrolysis of GDL and acid-alkali neutralizing reaction between GDL-COOH and shellac-Na. The FTIR spectroscopy was used to characterize the time-dependent gel processes. As the hydrolysis of GDL proceeded (Fig. 4B), the peak at 1745 cm−1 was transformed into two peaks located at 1758 cm−1 and 1745 cm−1, resulting from the hydrolytic cleavage of GDL lactone bond. In Fig. 4C, as the increase of the intensity of the new peak at 1781 cm−1, the intensity of the peak at 1745 cm−1 belonged to GDL decreased. This can indicate that the carboxyl group released from the hydrolytic cleavage of GDL lactone bond could exchange H+ ions with the Shellac-COONa to prepare the Shellac-COOH.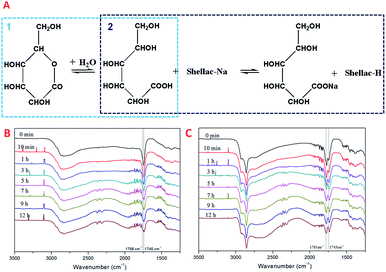 | ||
| Fig. 4 The proposed mechanism for the reaction in the shellac gel process (A), the FTIR spectroscopy of hydrolysis of GDL (B), the FTIR spectroscopy of the process of hydrogel (C). | ||
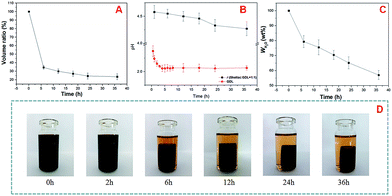
Fig. 5A showed that the volume of the shellac hydrogel (Fig. 5A) decreased in the gelation process, meanwhile, the water content (Fig. 5C) of the shellac hydrogel and the pH (Fig. 5B) of the gel system kept the same tendency. In the first 6 h, there was a major reduction in the volume (34.5%) and the water content (79.3%) of the shellac hydrogel, and then the changes became slow in the following process. The dramatic changes of the volume and the water content of the shellac hydrogel in the first 6 h were corresponding to the large pH decrease of GDL from 2.38 to 2.06, which was attributed to the H+ ions exchange from the GDL-COOH to shellac-COONa. After 24 h, the three dimensional parameters of the hydrogel tend to be stable as shown in Fig. 5C. So, the pH should be the key factor to induce the gel behavior.
Analysis of gel process of shellac hydrogel
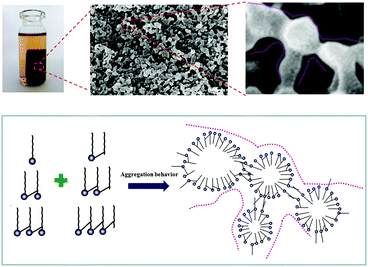
To analyze the gel process of shellac hydrogel, the microstructure of the shellac hydrogel was also be determined. The SEM images of the shellac hydrogel cross-section obtained at different time were showed in Fig. 6. It was found that the hydrogel was composed of nano-fibers. The appearance of the nano-fibers seemed like connected nano-size particles in series. Compared to Fig. 6A and E, with the gelation proceeding, the width of the nano-size particles changed from dozens of nanometers to hundreds of nanometers, which may be resulted from the aggregation of the shellac in the process. To prove the existence of the nano-particles, micro shellac hydrogel dispersion was fabricated by mixing the low concentration shellac-Na (0.01 wt%) aqueous solution with equal molar GDL.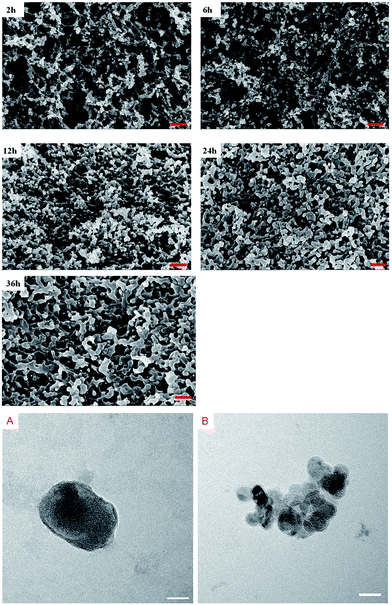 | ||
| Fig. 6 The SEM of the cross-section of the hydrogel obtained at different time (bar = 500 nm), the TEM image of the shellac vesicles (A and B, bar = 50 nm). | ||
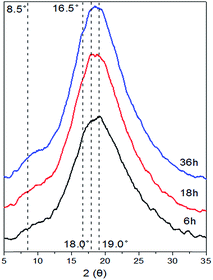
As Fig. 6A showed, we found that there indeed was nano-scale oval particle with several nanometers coating like vesicle, meanwhile some small nano-size shellac vesicles could aggregate together to assemble into larger scale continuous phase (Fig. 6B). The XRD patterns of the shellac hydrogel obtained at different time were displayed in Fig. 7. With the gelation proceeding, the intensity of the peak at 8.5° and 16.5° also increased, resulting from the aggregation of the shellac molecules corresponding to the growth of width of the shellac nano-size particle (Fig. 6). Based on the above, the mechanism of the shellac gelation process was described in Fig. 8. In the beginning, as the hydrolysis of GDL proceeded, the amphiphilic molecules shellac-COONa changed to shellac-COOH and assembled into the nano-size vesicles.
Subsequently, these vesicles made from shellac-COOH similar to asymmetric gemini surfactants could aggregate together to fabricate the network of the shellac hydrogel by the hydrophobic association between two sets of short hydrophobic chains from two adjacent vesicles.22,47
Characterization of the properties of the shellac hydrogel
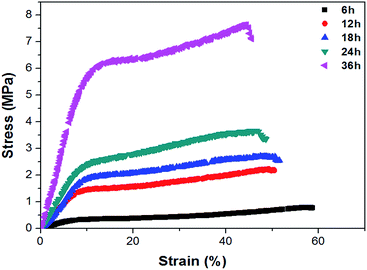
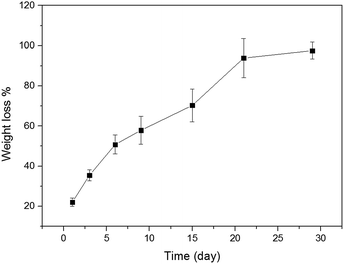
The shellac hydrogels should be given more attention to their mechanical properties. The typical compressive curves of the shellac hydrogels were shown in Fig. 9, the details of the compressive test were revealed in Table 2. As expected, the shellac hydrogel obtained at 6 h exhibited poor mechanical properties and its compressive fracture stress, strain and compress modulus were 0.8 MPa, 57.4%, 4.8 MPa respectively. With the aggregation of the shellac molecule on the nano-fibers of shellac hydrogel, the compressive fracture stress, strain and compress modulus of the shellac hydrogel increased, being 7.6 MPa, 43.7%, 61 MPa respectively. It is worth noting that the compressive curves showed that hydrogel obtained at 36 h owns high compress modulus reaching to 61 MPa and had a yield point, which was different from the ordinary hydrogel and similar to the polymer foams, such as polyurethane foam.48,49 This high mechanical performance and the yield point could be attributed to the compact microstructure of shellac hydrogel (Fig. 6) with low water content.As Fig. 10 showed, the degradation behavior of shellac hydrogel obtained at 36 h in PBS (pH = 7.2) was also tested at 37 °C. In the first week the loss weight ratio of shellac hydrogel exceeded 50%. After four weeks, its loss weight ratio reached 90%. Because of the low-molecular weight (Fig. 1 showed), the shellac hydrogel had exhibited obviously faster degradation rate than cellulose, chitin, protein and other biomacromolecules.50,51
Conclusions
In this work, we successfully developed a facile route to prepare shellac hydrogel by mixing the shellac-COONa and GDL, and meanwhile of the mechanism of the gelation process of shellac hydrogel was also investigated. We found that in the beginning of the gelation, as the hydrolysis of GDL processed, the amphiphilic molecules shellac-COONa changed into shellac-COOH and were assembled into nano-size vesicles. Then, these vesicles made from shellac-COOH similar to asymmetric gemini surfactants could aggregate to fabricate the network of the shellac hydrogel by the hydrophobic association between two sets of short hydrophobic chains from two adjacent vesicles. The shellac hydrogel had excellent mechanical properties and degradation performance which can be used as a new kind of soft material, such as high mechanical performance bio-based foams.Acknowledgements
This study was financially supported by National High Technology Research and Development Program of China (863 Program) (2014AA021801) and the Special Fund Project for the Scientific Research of State Forest Public Welfare Industry of China (201204602). We also acknowledged our sincere gratitude to all staff members of the department for their kind assistance.References
- O. P. Chauhan, P. S. Raju, A. Singh and A. S. Bawa, Food Chem., 2011, 126, 961–966 CrossRef CAS
.
- E. L. Hult, M. Iotti and M. Lenes, Cellulose, 2010, 17, 575–586 CrossRef CAS
.
- A. R. Patel, C. Remijn, A.-I. M. Cabero, P. C. M. Heussen, J. W. M. S. ten Hoorn and K. P. Velikov, Adv. Funct. Mater., 2013, 23, 4710–4718 CAS
.
- A. R. Patel, P. S. Rajarethinem, A. Gredowska, O. Turhan, A. Lesaffer, W. H. De Vos, D. Van de Walle and K. Dewettinck, Food Funct., 2014, 5, 645–652 CAS
.
- A. R. Patel, D. Schatteman, W. H. De Vos and K. Dewettinck, RSC Adv., 2013, 3, 5324–5327 RSC
.
- L. M. Bellan, M. Pearsall, D. M. Cropek and R. Langer, Adv. Mater., 2012, 24, 5187–5191 CrossRef CAS PubMed
.
- A. Singh, A. Upadhye, V. Mhaskar, S. Dev, A. Pol and V. Naik, Tetrahedron, 1974, 30, 3689–3693 CrossRef CAS
.
- M. Luangtana-anan, S. Limmatvapirat, J. Nunthanid, C. Wanawongthai, R. Chalongsuk and S. Puttipipatkhachorn, J. Agric. Food Chem., 2007, 55, 687–692 CrossRef CAS PubMed
.
- A.-L. Fameau, C. Gaillard, D. Marion and B. Bakan, Green Chem., 2013, 15, 341–346 RSC
.
- J. A. Heredia-Guerrero, J. J. Benítez and A. Heredia, BioEssays, 2008, 30, 273–277 CrossRef CAS PubMed
.
- J. J. Benítez, J. A. Heredia-Guerrero and A. Heredia, J. Phys. Chem. C, 2007, 111, 9465–9470 Search PubMed
.
- J. J. Benítez, J. A. Heredia-Guerrero, F. M. Serrano and A. Heredia, J. Phys. Chem. C, 2008, 112, 16968–16972 Search PubMed
.
- A.-L. Fameau, A. Arnould and A. Saint-Jalmes, Curr. Opin. Colloid Interface Sci., 2014, 19, 471–479 CrossRef CAS
.
- A.-L. Fameau, A. Arnould, M. Lehmann and R. von Klitzing, Chem. Commun., 2015, 51, 2907–2910 RSC
.
- A. L. Fameau, A. Carl, A. Saint-Jalmes and R. Von Klitzing, ChemPhysChem, 2015, 16, 66–75 CrossRef CAS PubMed
.
- A. L. Fameau, A. Saint-Jalmes, F. Cousin, B. Houinsou Houssou, B. Novales, L. Navailles, F. Nallet, C. Gaillard, F. Boué and J. P. Douliez, Angew. Chem., Int. Ed., 2011, 50, 8264–8269 CrossRef CAS PubMed
.
- J.-P. Douliez, L. Navailles, E. J. Dufourc and F. Nallet, Langmuir, 2014, 30, 5075–5081 CrossRef CAS PubMed
.
- A.-L. Fameau, F. Cousin, L. Navailles, F. D. R. Nallet, F. O. Boué and J.-P. Douliez, J. Phys. Chem. B, 2011, 115, 9033–9039 CrossRef CAS PubMed
.
- B. Novales, A. Riaublanc, L. Navailles, B. r. n. Houinsou Houssou, C. Gaillard, F. Nallet and J.-P. Douliez, Langmuir, 2010, 26, 5329–5334 CrossRef CAS PubMed
.
- A.-L. Fameau and T. Zemb, Adv. Colloid Interface Sci., 2014, 207, 43–64 CrossRef CAS PubMed
.
- A.-L. Fameau and A. Saint-Jalmes, Soft Matter, 2014, 10, 3622–3632 RSC
.
- F. M. Menger and A. V. Peresypkin, J. Am. Chem. Soc., 2003, 125, 5340–5345 CrossRef CAS PubMed
.
- N. Kumar, S. Varghese, G. Narayan and S. Das, Angew. Chem., Int. Ed., 2006, 45, 6317–6321 CrossRef CAS PubMed
.
- F. Xu, H. Wang, J. Zhao, X. Liu, D. Li, C. Chen and J. Ji, Macromolecules, 2013, 46, 4235–4246 CrossRef CAS
.
- W. Zhang, C. Yuan, J. Guo, L. Qiu and F. Yan, ACS Appl. Mater. Interfaces, 2014, 6, 8723–8728 CAS
.
- A. Barbetta, E. Barigelli and M. Dentini, Biomacromolecules, 2009, 10, 2328–2337 CrossRef CAS PubMed
.
- R. G. McGuire and D. A. Dimitroglou, Biocontrol Sci. Technol., 1999, 9, 53–65 CrossRef
.
- S. Y. Lee, K. L. Dangaran, J. X. Guinard and J. M. Krochta, J. Food Sci., 2002, 67, 2764–2769 CrossRef
.
- M. Irimia-Vladu, E. D. Glowacki, G. Schwabegger, L. Leonat, H. Z. Akpinar, H. Sitter, S. Bauer and N. S. Sariciftci, Green Chem., 2013, 15, 1473–1476 RSC
.
- Y. Singhbabu, S. K. Choudhary, N. Shukla, S. Das and R. K. Sahu, Nanoscale, 2015, 7, 6510–6519 RSC
.
- M. Luangtana-anan, J. Nunthanid and S. Limmatvapirat, J. Agric. Food Chem., 2010, 58, 12934–12940 CrossRef CAS PubMed
.
- K. Steigerwald, S. Merl, A. Kastrati, A. Wieczorek, M. Vorpahl, R. Mannhold, M. Vogeser, J. Hausleiter, M. Joner, A. Schömig and R. Wessely, Biomaterials, 2009, 30, 632–637 CrossRef CAS PubMed
.
- G. Vannuruswamy, G. V. N. Rathna, B. S. T. Gadgil and A. P. Gadad, J. Bioact. Compat. Polym., 2015, 30, 472–489 CrossRef CAS
.
- A. R. Patel, D. Schatteman, W. H. De Vos, A. Lesaffer and K. Dewettinck, J. Colloid Interface Sci., 2013, 411, 114–121 CrossRef CAS PubMed
.
- C. Coelho, R. Nanabala, M. Ménager, S. Commereuc and V. Verney, Polym. Degrad. Stab., 2012, 97, 936–940 CrossRef CAS
.
- H. S. Patel and S. J. Patel, J. Reinf. Plast. Compos., 2010, 29, 1267–1272 CrossRef CAS
.
- H. S. Patel and S. J. Patel, Chem.–Eur. J., 2010, 7, S55–S60 CAS
.
- A. L. Campbell, S. D. Stoyanov and V. N. Paunov, Soft Matter, 2009, 5, 1019–1023 RSC
.
- A. L. Campbell, S. D. Stoyanov and V. N. Paunov, ChemPhysChem, 2009, 10, 2599–2602 CrossRef CAS PubMed
.
- A. L. Campbell, B. L. Holt, S. D. Stoyanov and V. N. Paunov, J. Mater. Chem., 2008, 18, 4074–4078 RSC
.
- S. A. Hamad, S. D. Stoyanov and V. N. Paunov, Soft Matter, 2012, 8, 5069–5077 RSC
.
- J. Xue and Z. B. Zhang, J. Appl. Polym. Sci., 2009, 113, 1619–1625 CrossRef CAS
.
- A. Patel, P. Heussen, J. Hazekamp and K. P. Velikov, Soft Matter, 2011, 7, 8549–8555 RSC
.
- A. Teearu, S. Vahur, U. Haljasorg, I. Leito, T. Haljasorg and L. Toom, J. Mass Spectrom., 2014, 49, 970–979 CrossRef CAS PubMed
.
- A. Singh, A. Upadhye, V. Mhaskar and S. Dev, Tetrahedron, 1974, 30, 867–874 CrossRef CAS
.
- S. Bhattacharjee and S. Bhattacharya, Chem. Commun., 2015, 51, 6765–6768 RSC
.
- M. Irimia-Vladu, Chem. Soc. Rev., 2014, 43, 588–610 RSC
.
- C. Zhang and M. R. Kessler, ACS Sustainable Chem. Eng., 2015, 3, 743–749 CrossRef CAS
.
- H. J. Wang, M. Z. Rong, M. Q. Zhang, J. Hu, H. W. Chen and T. Czigány, Biomacromolecules, 2007, 9, 615–623 CrossRef PubMed
.
- Q. Shi, C. Zhou, Y. Yue, W. Guo, Y. Wu and Q. Wu, Carbohydr. Polym., 2012, 90, 301–308 CrossRef CAS PubMed
.
- H. Nagahama, N. Nwe, R. Jayakumar, S. Koiwa, T. Furuike and H. Tamura, Carbohydr. Polym., 2008, 73, 295–302 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |