Electrical characterization and conductivity optimization of laser reduced graphene oxide on insulator using point-contact methods
Abstract
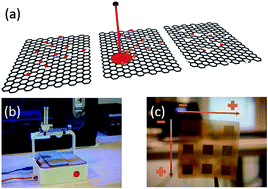
The present work is focused on the electrical characterization of laser-assisted reduced graphene oxide by point-contact techniques. The aim is twofold: firstly, the careful investigation of in-line two and four point-contact techniques applied to macroscopic samples of reduced graphene oxide. The combination of both methods has shed light on the role of the point-contact when extracting the intrinsic resistivity of the material. Secondly, once the measurement protocol is well understood, it is applied to improve the conductivity of the samples by the adjustment of the initial colloid concentration and the photothermal power intensity used for the reduction. The final optimized samples present promising conductivity, comparable to that of large graphene sheets obtained by chemical vapor deposition methods.


 Please wait while we load your content...
Please wait while we load your content...