Alkylguanidinium based ionic liquids in a screening study for the removal of anionic pollutants from aqueous solution†
Abstract
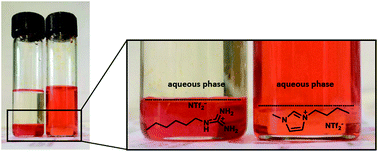
Monoalkylguanidinium bis-trifluoromethane sulfonimides are water immiscible functional ionic liquids which appear as highly efficient phases for the sequestration of anionic pollutants from aqueous solutions. The new compounds show significantly enhanced extraction efficiency compared to conventional imidazolium based ionic liquids.


 Please wait while we load your content...
Please wait while we load your content...