One-pot synthesis and loading of mesoporous SiO2 nanocontainers using micellar drugs as a template†
Abstract
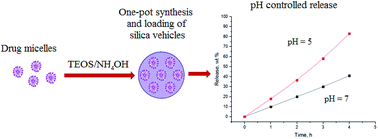
A new approach is proposed for creating of pH-sensitive mesoporous silica nanocontainers with ultrahigh capacity for amphiphilic functional compounds. It relies on the use of micelles of such compounds as templates for the sol–gel synthesis of SiO2 nanoparticles. The possibility of such one-stage synthesis and loading of SiO2 nanocontainers has been demonstrated using the micelles of the bactericidal drug Myramistin as a template.

 Please wait while we load your content...
Please wait while we load your content...