Exploring the interaction of L-cysteine capped CuS nanoparticles with bovine serum albumin (BSA): a spectroscopic study
S. Prasanth,
D. Rithesh Raj,
T. V. Vineeshkumar,
Riju K. Thomas and
C. Sudarsanakumar*
School of Pure and Applied Physics Mahatma Gandhi University, Kottayam, Kerala, India 686560. E-mail: c.sudarsan.mgu@gmail.com; Fax: +91-481-2730423; Tel: +91-481-2731043 Tel: +91-9447141561
First published on 13th June 2016
Abstract
The interactions between nanoparticles and proteins have prime importance in nanomedicine and nanotoxicology. L-Cysteine capped copper sulfide (Cy-CuS) nanoparticles having average particle size of 6 nm have been synthesized using chemical coprecipitation method and their interactions with Bovine Serum Albumin (BSA) were explored using various spectroscopic techniques. A ground state complex is formed between BSA and Cy-CuS nanoparticles in solution. The number of binding sites, binding constant, quenching constant and the binding distance between Cy-CuS nanoparticles and BSA were determined. The overall conformation of BSA in the complex was unaltered, however the α-helical content of BSA was marginally reduced.
1. Introduction
Nanostructured materials are significant in almost all branches of science and engineering due to their tunable optical, electronic, and magnetic properties arising from the quantum confinement effects. The transition metal compounds have received considerable attention because of their excellent properties. The plasmonic behaviour of transition metal chalcogenide nanoparticles enables them to emerge as functional nanoscale entities with potential applications in catalysis, energy storage and conversion, biomedical research, and optoelectronic devices.1–6 Among the transition metal chalcogenides copper sulfide (CuS) is extensively used in many biological fields such as in drug delivery, photothermal therapy, and in vitro bio sensing.7–11 CuS, a p-type semiconductor with excellent optical and electrical properties is known to form a variety of stoichiometric compounds with varying band gap.12Understanding the interactions of nanoparticles with proteins is a matter of concern for their effective use in biological and medical applications. The interactions may alter or perturb the protein conformation and these conformational changes could induce unexpected biological reactions leading to toxicity.13 Bovine Serum Albumin (BSA), is a well-studied medically important basic protein of blood plasma. It is highly stable and structurally homologous to human serum albumin.14,15 BSA has several functions such as maintaince of the colloidal osmotic blood pressure and pH, transport of drugs and nutrition through human body, delivery of fatty acids, etc.16
The interactions of BSA with various nanoparticles such as Ag, CdS, Au, ZnO, Al2O3, carbon nanotubes and TiO2 have been reported.17–22 However, the interactions of L-cysteine capped CuS nanoparticles with BSA have not been reported so far.
This work is an investigation of the interactions of L-cysteine capped CuS nanoparticles (Cy-CuS NPs) with BSA by spectroscopic methods like UV-visible absorption, fluorescence and circular dichroism (CD). The fluorescence quenching mechanism, number of binding sites, binding constant and binding distance involved in the interactions has been estimated. Fluorimetry is an extensively used technique to study the binding interactions because of its sensitivity, convenience to use, and the valuable informations that can be deduced. The present study provides a better understanding of how Cy-CuS nanoparticles behave in the biological milieu.
BSA consists of three domains (I, II & III) and each domain is divided in to two subdomains (A & B) and are connected by 17 disulfide bonds. The three intrinsic fluorophores of BSA such as tryptophan, tyrosine, and phenylalanine are highly sensitive to their micro environment. Among the three fluorophores tryptophan contributes highest to the fluorescence of BSA.22–25 BSA has two tryptophan residues, Trp-212 and Trp-134. Trp-134 is located on the hydrophilic environment near the surface of the protein and Trp-212 is in the hydrophobic environment within the protein (Scheme 1). Trp-212 contributes more to the fluorescence intensity than Trp-134.26,27
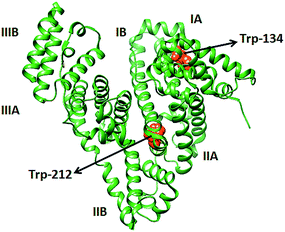 | ||
| Scheme 1 Cα trace of BSA (PDB no. 4F5C) generated by Chimera software and the relative position of two tryptophan residues are marked. | ||
2. Experimental
2.1. Materials
Copper acetate monohydrate (Cu(CH3COO)2) and thiourea (NH2SNH2) were purchased from Merck. L-Cysteine (C3H7NO2S) and bovine serum albumin were purchased from Sigma Aldrich. BSA was dissolved in milli pore water to prepare stock solution and stored at 0–4 °C. All measurements were performed at ambient temperature.2.2. Instrumentation
X-ray diffraction pattern was recorded using PANalytical X-ray diffractometer with CuKα radiation (λ = 1.5406 Å) in the range of 10–80° (2θ) at a scanning rate of 0.01° min−1. The size and morphology of the material was investigated using transmission electron microscope (TEM) (JEOL JEM 2100 with LaB6 filament). UV-vis absorption studies were carried out using Shimadzu 2401 UV-vis spectrophotometer. The FT-IR spectrum was measured on a Shimadzu Model: IR Prestige 21, with ZnSe ATR crystal (Pike technologies) spectrometer. The photoluminescence (PL) spectra were measured on a Fluoromax-4 Spectrophotometer. Fluorescence lifetime measurements were carried out at an excitation wavelength of 280 nm using a picosecond diode (Spectra-NanoLED source S-278). Circular dichroism (CD) data was collected from JASCO 810 circular dichroism spectropolarimeter using quartz cuvette of 1 cm path length.2.3. Preparation procedure
Stock solution of BSA (0.1%) was prepared in millipore water by dissolving 0.1 g of BSA in 100 mL of water and used for further studies. Interactions of BSA with CuS nanoparticles were carried out in millipore water. Cy-CuS NPs at various concentrations (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 mM) were allowed to interact with a constant (0.1%) concentration of BSA for 20 minutes at room temperature (Scheme 2).
3. Results and discussion
3.1. Characterization of CuS nanoparticles
The TEM image and selected area electron diffraction (SAED) pattern of Cy-CuS NPs are shown in Fig. 1a and b respectively, confirming the presence of spherical CuS grains with average size of 6 nm. The nanoparticles tend to form small clusters because of the attraction of their surface groups.X-ray diffraction pattern (XRD) exhibits crystallinity of Cy-CuS NPs and is shown in Fig. 1c. The broadening of the peaks indicates the nanocrystalline nature of the sample. The four diffraction peaks corresponding to (102), (103), (110), and (116) planes confirm the hexagonal crystal structure of the particles (JCPDS 060464). The average crystalline size (7 nm) was estimated from the Debye–Scherrer formula.30,31
The presence of cysteine on the surface of CuS nanoparticles was examined from Fourier Transform Infrared (FTIR) spectrum (Fig. 1d). The peak observed at 3142 cm−1 corresponds to the stretching vibrations of the NH2 group in cysteine.32,33 The asymmetric and symmetric stretching vibrations of the carboxyl group (COO−) are observed at 1666 cm−1 and 1427 cm−1 respectively34 and the peak at 1066 cm−1 corresponds to the C–H bending vibration.35,36 These results suggest the successful capping of cysteine on the surface of CuS nanoparticles.
3.2. Absorption characteristics of BSA and BSA–CuS
The absorption spectra of pure BSA and BSA with various concentrations of Cy-CuS NPs were analysed and are shown in Fig. 2. The spectra show a strong absorption band in the UV region with an absorption maximum at 278 nm, attributed to the π–π* transition of the aromatic amino acid residues tyrosine and tryptophan.37 On increasing the concentration of Cy-CuS NPs, the absorbance of BSA increases gradually. The interactions of BSA on the surface of Cy-CuS NPs lead to a ground state BSA–CuS complex and hence the absorbance of BSA increases progressively with the concentration of Cy-CuS NPs. Similar interactions of BSA with capped CdS, ZnO CdTe, Al2O3 and TiO2 have been previously reported.38,39 | ||
| Fig. 2 Absorption spectra of BSA and BSA–Cy-CuS NPs at various concentrations. The absorption peak at 278 nm increases with increase in concentration of the Cy-CuS NPs. | ||
With the addition of increasing concentration of nanoparticles to BSA, the microenvironment around the tryptophan residues changes. Trp-212 which is located on the hydrophobic pocket of BSA is exposed to the aqueous medium up to a certain degree. The Cy-CuS NPs were bind to BSA and the secondary structure of BSA was altered slightly.22
3.3. Fluorescent quenching studies of BSA and BSA–Cy-CuS nanoparticles
The fluorescence emission spectra of bare BSA and BSA–CuS (0.1–0.7 mM) nanoparticles were recorded at room temperature. BSA shows an emission band at 350 nm for an excitation wavelength of 280 nm (Fig. 3a). It is found that the fluorescence intensity of BSA decreases gradually with increasing concentration of CuS nanoparticles. The quenching of BSA emission intensity by Cy-CuS NPs can be described by Stern–Volmer equation.40–43| F0/F = 1 + Ksv[Q] | (1) |
3.4. The binding constant and the number of binding sites in BSA–Cy-CuS NPs complex
The fluorescence quenching data provides information about the binding constant (K), the number of binding sites (n) and the values can be evaluated from the equation.44,45
 | (2) |
Fig. 4 shows the plot of  versus log[Q]. The values of n and K are obtained from the slope and Y intercept respectively and the corresponding values are 3.6362 × 102 M−1 and 1.177.
versus log[Q]. The values of n and K are obtained from the slope and Y intercept respectively and the corresponding values are 3.6362 × 102 M−1 and 1.177.
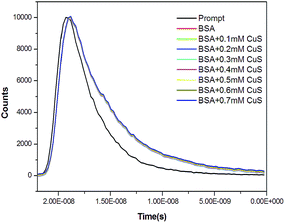
3.5. Time resolved measurements
The mechanism of fluorescence emission quenching of tryptophan by Cy-CuS NPs may be due to static or dynamic. The measurement of fluorescence lifetime is one of the absolute methods to distinguish between static and dynamic quenching.18,45 The fluorescence lifetime of pure BSA and BSA with various concentrations (0.1–0.7 mM) of Cy-CuS nanoparticles (Fig. 5) were investigated. The decay curves of BSA and BSA–Cy-CuS NPs complex were fitted to multiexponential functions. Here, BSA requires three time constants for satisfactory fits: τ1 = 2.178 ns (8.61%), τ2 = 5.418 ns (62.47%), and τ3 = 7.792 ns (29.12%). The mean life time value can be calculated using the equation,| τm = τ1a1 + τ2a2 + τ3a3 | (3) |
The mean life time of BSA obtained from the time resolved measurement is 5.831 ns and does not change significantly with the addition of CuS nanoparticles (0.1–0.7 mM). This implies that the quenching is static in nature and the interactions lead to a non-fluorescent ground state complex. The decay parameters are summarized in Table 1.
| Concentration of Cy-CuS (10−4 M) | τ1 (ns) | τ2 (ns) | τ3 (ns) | a1 | a2 | a3 | τm (ns) | χ2 |
|---|---|---|---|---|---|---|---|---|
| 0 | 2.178 | 5.418 | 7.792 | 8.61 | 62.27 | 29.12 | 5.831 | 1.218 |
| 1 | 0.888 | 4.411 | 7.162 | 2.62 | 42.33 | 55.05 | 5.833 | 1.326 |
| 2 | 0.807 | 3.887 | 6.792 | 2.22 | 27.83 | 69.95 | 5.851 | 1.228 |
| 3 | 2.966 | 6.373 | 14.836 | 16.93 | 81.73 | 1.34 | 5.909 | 1.270 |
| 4 | 2.309 | 5.918 | 10.082 | 9.61 | 82.52 | 7.87 | 5.899 | 1.154 |
| 5 | 2.957 | 6.362 | 13.284 | 17.32 | 81.29 | 1.39 | 5.868 | 1.212 |
| 6 | 2.673 | 6.226 | 11.699 | 14.13 | 83.32 | 2.55 | 5.863 | 1.172 |
| 7 | 7.001 | 24.010 | 18.603 | 65.58 | 4.10 | 10.36 | 5.811 | 1.232 |
3.6. TEM characterization of BSA–Cy-CuS nanoparticles
To ensure the binding of Cy-CuS NPs on BSA, TEM image of BSA with 0.7 mM Cy-CuS NPs was taken (Fig. 6). The corona formation can be seen from the TEM images confirming the strong binding of Cy-CuS NPs on to BSA.3.7. Binding distance between BSA and Cy-CuS nanoparticles
The distance between CuS nanoparticles and amino acid residues of BSA can be determined from Forster non-radiative energy transfer (FRET). This can happen only at the following conditions; the donor can emit fluorescence, the fluorescence emission spectrum of the donor overlaps with the UV absorption spectrum of the acceptor and the distance between the donor and the acceptor is less than 7 nm.46 The distance between the acceptor and the donor can be determined from the energy transfer efficiency E. The critical energy-transfer distance R0 and E are connected by the relation
 | (4) |
| R06 = 8.8 × 10−25k2N−4ϕJ | (5) |
 | (6) |
 | (7) |
The overlap of the absorption spectrum of Cy-CuS NPs with the fluorescence emission spectrum of BSA is shown in Fig. 7. The value of J, calculated by integrating the spectra in the range 300–500 nm is 1.738 × 10−15 cm3 L mol−1. When k2 = 2/3, N = 1.36 and ϕ = 0.41,48 the critical distance R0 calculated from eqn (5) is 2.58 nm. The energy transfer efficiency E calculated using eqn (7) is 0.0926. Therefore, the binding distance, r between Cy-CuS nanoparticles and the amino acid residue in BSA is found to be 3.77 nm.
3.8. Circular dichroism spectroscopy
The far-UV CD spectra of BSA were analysed in the presence of Cy-CuS nanoparticles to understand the specific changes in the secondary structure of BSA upon binding with Cy-CuS NPs.49,50 The Fig. 8 shows the CD spectra of BSA in the absence and presence of Cy-CuS NPs. The negative bands at 209 nm and 222 nm in the ultraviolet region of the BSA CD spectrum are characteristics of the α-helical structure of BSA protein.51,52 The band at 209 nm is originating from π–π* transfer of peptide bond and the band at 222 nm is from n–π* transfer of peptide bond of the α-helix.53 A clear decrease in the band intensity with the addition of Cy-CuS NPs, indicates a fall in α-helical content occurring due to the binding of Cy-CuS NPs with the amino acid residues in BSA.The α-helical content of pure BSA and BSA with various concentrations of nanoparticles can be calculated using the following equation
 | (8) |
 | (9) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 is the MRE value of a pure α-helix at 209 nm and 4000 is the MRE of the β-form and random coil conformation cross at 209 nm.54 The % variations of α-helix in BSA in the presence of Cy-CuS NPs are calculated using the above equations and the values are depicted in Table 2.
000 is the MRE value of a pure α-helix at 209 nm and 4000 is the MRE of the β-form and random coil conformation cross at 209 nm.54 The % variations of α-helix in BSA in the presence of Cy-CuS NPs are calculated using the above equations and the values are depicted in Table 2.
| Concentration of Cy-CuS NPs (10−4 M) | α-Helix content (209 nm)% of BSA |
|---|---|
| 0 | 48.9 |
| 1 | 47.7 |
| 3 | 46.3 |
| 5 | 45.2 |
The binding of Cy-CuS NPs with BSA induces minor conformational changes in BSA. The decrease in the α-helical content (∼3.7%, Table 2) is due to partial unfolding of the peptide strands in BSA upon interactions with Cy-CuS NPs.55–58 This variation is very small when compared with other nanoparticles such as Al2O3, carbon nanotubes, and FeO2.59 Hence Cy-CuS NPs are promising candidates for clinical applications.
The two tryptophan residues (Trp-134 and Trp-212) in BSA are located at two separate domains. Trp-134 is located on the hydrophilic pocket on the surface of BSA in the second helix of the subdomain IB and Trp-212 is in the hydrophobic pocket of subdomain IIA. The interactions between nanoparticles and proteins are highly dependent on the size and surface of nanoparticles. There are mainly three different types of molecular interactions such as electrostatic, hydrophobic, and hydrogen bonding. Hydrophobic interaction leads to the binding of the nanoparticles at the hydrophobic pocket near Trp-212 while electrostatic and hydrogen bonding interactions lead to the binding of nanoparticles near Trp-134 residue.60,61 A schematic representation of the possible interactions is given in Scheme 3.
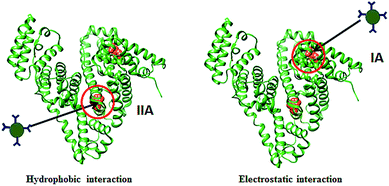 | ||
| Scheme 3 Schematic representation of hydrophobic and electrostatic interactions between Cy-CuS NPs and BSA. | ||
The hydrophobic interactions between ZnO and Au nanoparticles with BSA were studied by S. Chakraborti et al.27,62 In both the cases the nanoparticles were bound in BSA subdomain IIA. S. Bhuiya and co-workers investigated the interactions of anticancer alkaloid chelerythrine with BSA using spectroscopic techniques. Chelerythrine binds in the BSA subdomain IIA where the interactions are hydrophobic and electrostatic. From the FRET studies the average distance between BSA and chelerythrine is <3 nm. The lower r value indicates closer distance between Trp-212 and chelerythrine and therefore the drug is proposed to bind near Trp-212.63 The interactions between Ag+ and BSA were reported by X. Zhao and co-workers, where the binding distance between donor and receptor was about 10.0 nm. The relatively longer distance indicates the possible site of interaction near Trp-134 located at the surface of BSA.64
In the present investigation when Cy-CuS NPs were added to BSA, the fluorescence quenching is observed on increasing the concentration of nanoparticles. The nature of interactions between BSA and Cy-CuS NPs is a decisive factor in the binding position of NPs, either to Trp-134 or to Trp-212. The exact binding location of Cy-CuS NPs in BSA could not be ascertained. But reasonable prediction about the binding position can be made from the FRET study which shows that the average distance between Cy-CuS NPs and protein is about 3.77 nm. This indicates the binding of the nanoparticles closer to Trp-212 (ref. 59 and 64) there by disturbing its hydrophobic environment and exposing to the surroundings.
4. Conclusions
Cysteine capped CuS nanoparticles were prepared by chemical coprecipitation and their interactions with BSA were studied by spectroscopic methods. Our results indicate that Cy-CuS NPs binds to BSA forming a complex leading to quenching of BSA fluorescence intensity and the static quenching mechanism was confirmed by fluorescence lifetime measurements. The quenching rate constant, binding constant, number of binding sites, and binding distance were calculated from the relevant fluorescence data. FRET study reveals the binding of Cy-CuS NPs near theTrp-212 located in the hydrophobic pocket of BSA. CD spectra indicate partial unfolding of α-helical peptide strands in BSA due to its interaction with Cy-CuS nanoparticles. The complex formation does not significantly influence the overall conformation of BSA. The current investigations will certainly strengthen our understanding of the biocompatibility of Cy-CuS nanoparticles. In summary, Cy-CuS nanoparticles are proper candidate for biomedical applications on account of minimal changes in the BSA conformation and hence in its biological activity.Acknowledgements
The authors S. P., D. R. R. and R. K. T. acknowledge UGC for BSR-RFSMS fellowship and authors acknowledge Mr Mohamed Hifsudheen, CSIR NIIST, Trivandrum for providing the instrumental facilities (Circular Dichroism).References
- J. S. Chung and H.-J. Sohn, J. Power Sources, 2002, 108, 226 CrossRef CAS.
- A. E. Raevskaya, A. L. Stroyuk, S. Y. Kuchmii and A. I. Kryukov, J. Mol. Catal. A: Chem., 2004, 212259 Search PubMed.
- A. A. Sagade and R. Sharma, Sens. Actuators, B, 2008, 133135 Search PubMed.
- S. R. Saptarshi, A. Duschl and A. L. Lopata, J. Nanobiotechnol., 2013, 11, 1 CrossRef PubMed.
- M. Antoniadou, V. M. Daskalaki, N. Balis, D. I. Kondarides, C. Kordulis and P. Lianos, Appl. Catal., B, 2011, 107, 188 CrossRef CAS.
- S. V. Bagul, S. D. Chavhan and R. Sharma, J. Phys. Chem. Solids, 2007, 68, 162 CrossRef.
- S. Goel, F. Chen and W. Cai, Small, 2014, 10, 631 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889 CrossRef CAS PubMed.
- S. Ramadan, L. Guo, Y. Li, B. Yan, W. L. Ramadan, L. Guo, Y. Li, B. Yan and W. Lu, Small, 2012, 8, 3143 CrossRef CAS PubMed.
- S. B. Lakshmanan, X. Zou, M. Hossu, L. Ma, C. Yang and W. Chen, J. Biomed. Nanotechnol., 2012, 8, 8883 Search PubMed.
- C. Ding, Z. Wang, H. Zhong and S. Zhang, Biosens. Bioelectron., 2010, 25, 1082 CrossRef CAS PubMed.
- P. L. Saldanha, R. Brescia, M. Prato, H. Li and M. Povia, Chem. Mater., 2014, 26, 1442 CrossRef CAS.
- T. Sen, S. Mandal, S. Haldar, K. Chattopadhyay and A. Patra, J. Phys. Chem. C, 2011, 115, 24037 CAS.
- S. Pramanik, P. Banerjee, A. Sarkar and S. C. Bhattacharya, J. Lumin., 2008, 128, 1969 CrossRef CAS.
- X. Zhao, F. Sheng, J. Zheng and R. Liu, J. Agric. Food Chem., 2011, 59, 7902 CrossRef CAS PubMed.
- A. Jhonsi, A. Kathiravan and R. Renganathan, Colloids Surf., B, 2009, 72, 167 CrossRef PubMed.
- S. P. Boulos, T. A. Davis, J. A. Yang, S. E. Lohse, A. M. Alkilany, L. A. Holland and C. J. Murphy, Langmuir, 2013, 29, 14984 CrossRef CAS PubMed.
- A. Bhogale, N. Patel, P. Sarpotdar, J. Mariam, P. M. Dongre, A. Miotello and D. C. Kothari, Colloids Surf., B, 2013, 102, 257 CrossRef CAS PubMed.
- O. V. Salata, J. Nanobiotechnol., 2004, 2, 1 CrossRef PubMed.
- A. Jhonsi, A. Kathiravan and R. Renganathan, Colloids Surf., B, 2009, 72, 167 CrossRef PubMed.
- S. Pramanik, P. Banerjee, A. Sarkar and S. C. Bhattacharya, J. Lumin., 2008, 128, 1969 CrossRef CAS.
- X. Zhao, R. Liu, Z. Chi, Y. Teng and P. Qin, J. Phys. Chem. B, 2010, 114, 5625 CrossRef CAS PubMed.
- Z. Xu, X.-W. Liu, Y.-S. Ma and H.-W. Gao, Environ. Sci. Pollut. Res. Int., 2010, 17, 798–806 CrossRef CAS PubMed.
- M. Dockal, J. Biol. Chem., 2000, 275, 3042 CrossRef CAS PubMed.
- Y. J. Hu, Y. Liu, R. M. Zhao, J. X. Dong and S. S. Qu, J. Photochem. Photobiol., A, 2006, 17, 9324 Search PubMed.
- L. Zhao, R. Liu, X. Zhao, B. Yang, C. Gao, X. Hao and Y. Wu, Sci. Total Environ., 2009, 407, 5019 CrossRef CAS PubMed.
- S. Chakraborti, P. Joshi, D. Chakravarty, V. Shanker, Z. A. Ansari, S. P. Singh and P. Chakrabarti, Langmuir, 2012, 2012(28), 11142 CrossRef PubMed.
- K. A. Ann Mary, N. V. Unnikrishnan and R. Philip, APL Mater., 2014, 2, 076104 CrossRef.
- A. Dutta and S. K. Dolui, Mater. Chem. Phys., 2008, 112, 448 CrossRef CAS.
- T. V. Vineeshkumar, D. Rithesh Raj, S. Prasanth, N. V. Unnikrishnan, R. Philip and C. Sudarsanakumar, Opt. Mater., 2014, 37, 439 CrossRef CAS.
- D. Rithesh Raj, S. Prasanth, T. V. Vineeshkumar and C. Sudarsanakumar, Opt. Commun., 2015, 340, 86 CrossRef CAS.
- A. Barth, Prog. Biophys. Mol. Biol., 2001, 74, 141 CrossRef.
- X. Liu, B. Li, F. Fu, K. Xu, R. Zou, Q. Wang, B. Zhang, Z. Chen and J. Hu, Dalton Trans., 2014, 43, 11709 RSC.
- A. Pawlukojć, J. Leciejewicz, A. J. Ramirez-Cuesta and N. Scheibe, Spectrochim. Acta, Part A, 2005, 61, 2474 CrossRef PubMed.
- Y.-S. Wei, S.-Y. Lin, S.-L. Wang, M.-J. Li and W.-T. Cheng, Biopolymers, 2003, 72, 345 CrossRef CAS PubMed.
- S. Prasanth, M. Varughese, N. Joseph, P. Mathew, T. K. Manojkumar and C. Sudarsanakumar, J. Mol. Struct., 2015, 1081, 366 CrossRef CAS.
- A. Rajeshwari, S. Pakrashi, S. Dalai, V. Iswarya, N. Chandrasekaran and A. Mukherjee, J. Lumin., 2014, 145, 859 CrossRef CAS.
- M. Idowu, E. Lamprecht and T. Nyokong, J. Photochem. Photobiol., A, 2008, 198, 7 CrossRef CAS.
- C. A. Haynes and W. Norde, J. Colloid Interface Sci., 1995, 169, 313 CrossRef CAS.
- J.-Q. Tong, F.-F. Tian, Q. Li, L.-L. Li, C. Xiang, Y. Liu, J. Dai and F.-L. Jiang, Photochem. Photobiol. Sci., 2012, 11, 1868 CAS.
- X. Wang and O. S. Wolfbeis, Chem. Soc. Rev., 2014, 43, 3666 RSC.
- F.-F. Tian, J.-H. Li, F.-L. Jiang, X.-L. Han, C. Xiang, Y.-S. Ge, L.-L. Li and Y. Liu, RSC Adv., 2012, 2, 501 RSC.
- S. Prasanth, P. Irshad, D. R. Raj, T. V. Vineeshkumar, R. Philip and C. Sudarsanakumar, J. Lumin., 2015, 166, 167 CrossRef CAS.
- G.-D. Yang, C. Li, A.-G. Zeng, Y. Zhao, R. Yang and X.-L. Bian, J. Pharm. Anal., 2013, 3, 200 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Plenum Press, NewYork, 1983, p. 258 Search PubMed.
- Y.-L. Wu, F. He, X.-W. He, W.-Y. Li and Y.-K. Zhang, Spectrochim. Acta, Part A, 2008, 71, 1199 CrossRef PubMed.
- S. Laib and S. Seeger, J. Fluoresc., 2004, 14, 187 CrossRef CAS PubMed.
- T. Sen, S. Mandal, S. Haldar, K. Chattopadhyay and A. Patra, J. Phys. Chem. C, 2011, 115, 24037 CAS.
- X. Zhang, L. Li, Z. Xu, Z. Liang, J. Su, J. Huang and B. Li, PLoS One, 2013, 8, 1 Search PubMed.
- B. Ahmad, S. Parveen and R. H. Khan, Biomacromolecules, 2006, 7, 1350 CrossRef CAS PubMed.
- B. Ahmad and L. J. Lapidus, J. Biol. Chem., 2012, 287, 9193 CrossRef CAS PubMed.
- L. Treuel, M. Malissek, J. S. Gebauer and R. Zellner, ChemPhysChem, 2010, 11, 3093 CrossRef CAS PubMed.
- S. Veeralakshmi, S. Nehru, S. Arunachalam, P. Kumar and M. Govindaraju, Inorg. Chem. Front., 2014, 1, 393 RSC.
- M. Mathew, S. Sreedhanya, P. Manoj, C. T. Aravindakumar and U. K. Aravind, J. Phys. Chem. B, 2014, 118, 3832 CrossRef CAS PubMed.
- C. Zheng, H. Wang, W. Xu, C. Xu, J. Liang and H. Han, Spectrochim. Acta, Part A, 2014, 118, 897 CrossRef CAS PubMed.
- I. Matei, A. M. Ariciu, M. V. Neacsu, A. Collauto, A. Salifoglou and G. Ionita, J. Phys. Chem. B, 2014, 118, 11238 CrossRef CAS PubMed.
- C. H. Yu, A. Al-Saadi, S.-J. Shih, L. Qiu, K. Y. Tam and S. C. Tsang, J. Phys. Chem. C, 2009, 113, 537 CAS.
- Q. Yang, J. Liang and H. Han, J. Phys. Chem. B, 2010, 113, 10454 CrossRef PubMed.
- S. Chatterjee and T. K. Mukherjee, Phys. Chem. Chem. Phys., 2014, 16, 8400 RSC.
- X. Zhao, F. Hao, D. Lu, W. Liu, Q. Zhou and G. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 18880 CAS.
- S. Chakraborty, P. Joshi, V. Shanker, Z. A. Ansari, S. P. Singh and P. Chakrabarti, Langmuir, 2011, 27, 7722 CrossRef CAS PubMed.
- X. Zhao, R. Liu, Y. Teng and X. Liu, Sci. Total Environ., 2011, 409, 892 CrossRef CAS PubMed.
- S. Bhuiya, A. B. Pradhan, L. Haque and S. Das, J. Phys. Chem. B, 2016, 120, 5 CrossRef CAS PubMed.
- X. Zhao, D. Lu, F. Hao and R. Liu, J. Hazard. Mater., 2015, 292, 98 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |