DOI:
10.1039/C6RA03556F
(Paper)
RSC Adv., 2016,
6, 23489-23497
A cascade reaction for the new and direct synthesis of indolofuroquinoxalines†
Received
7th February 2016
, Accepted 19th February 2016
First published on 22nd February 2016
Abstract
The 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives are synthesized directly from methyl 2-(2-chloro-1H-indol-3-yl)-2-oxoacetate or its N-alkyl derivatives under neutral or mildly acidic conditions. This new one-pot methodology was found to be general and greener as it avoids the use of environmentally harmful POCl3 and strong alkali required for the previously reported method. It is also amenable for scale-up.
Introduction
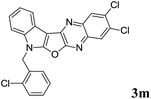
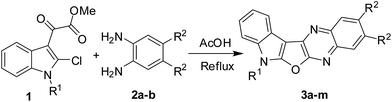
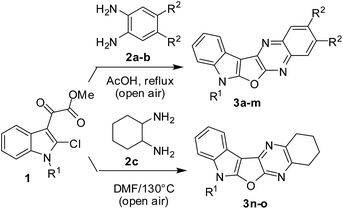
Prompted by the reported pharmacological properties of several polynuclear fused N-heteroarenes such as antiviral agent B-220, and antitumor agents NCA0424 and NCA0465 (all belong to the indolo[2,3-b]quinoxaline class),1 we synthesized a series of compounds (A, Fig. 1) containing an indole ring fused with pyrrolo-, furo- and thieno[2,3-b]quinoxaline moieties.2 While several of these compounds showed growth inhibition of lung, breast and cervical cancer cells, further in vitro studies of these compounds revealed compound B (Fig. 1) as an initial hit. Compound B showed 75–85% inhibition (better than other compounds) when tested against these cancer cell lines indicating favorable pharmacological effect of furan moiety over other 5-membered ring present between the indole and quinoxaline moiety. Notably, due to the structural resemblance with DNA intercalators such as antineoplastic agent ellipticine the mode of action of this class of compounds could to be based mainly on DNA intercalation, inhibition of topoisomerase II, and formation of covalent DNA adducts.3 Nevertheless, in continuation of this research we required a library of molecules based on the structural framework of B for detailed Structure Activity Relationship (SAR) studies. In our previous effort only two molecules based on B were prepared2 and the earlier method used for this purpose involved treatment of dichloro compound (C) with a strong alkali such as NaOH at an elevated temperature (Scheme 1). Moreover, preparation of starting compound C was cumbersome as it involved the use of environmentally harmful POCl3 at 100–110 °C. Recently, we have observed that these reaction conditions are not necessary for the synthesis of compound B and its derivatives. Instead, treatment of chloro compound 1 [i.e. methyl 2-(2-chloro-1H-indol-3-yl)-2-oxoacetate or its N-alkyl derivatives obtained from indolin-2-one]4 with aliphatic or aromatic 1,2-diamines (2) can afford the desired 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives (3 i.e. B or its analogues) directly either under neutral or mild acidic conditions (Scheme 2). The present methodology certainly has advantages over the previously reported method as it avoids the cumbersome preparation of starting material C and the use of strong alkali during the conversion of C to B. Moreover, the present approach based on a cascade reaction is a single-step method whereas several steps were involved in the previous synthesis of B.2 Herein we report results of our recent study.
 |
| | Fig. 1 Reported bioactive indolo[2,3-b]quinoxaline derivatives (B-220, NCA0424 and NCA0465) and compounds (A and B) reported by us.2 | |
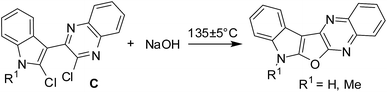 |
| | Scheme 1 Previous synthesis of compound B and its analogue.2 | |
 |
| | Scheme 2 New synthesis of 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives (3). | |
Results and discussions
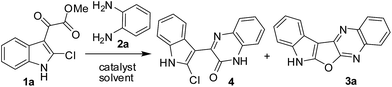
To establish the optimum reaction conditions for the new synthesis of compound 3 we choose the reaction of 1a with 2a as a model reaction. This reaction was performed under various conditions (Table 1). Initially, the use of a range of acid catalysts e.g. H2SO4, TFA, PTSA and AcOH was examined in two solvents e.g. EtOH and toluene (entries 1–8, Table 1). While reactions proceeded in these cases but afforded a mixture of two products e.g. the desired product 3a and the uncyclized compound 4. Indeed, the compound 4 was isolated in higher yields than 3a in all these cases. It should be noted that the compound 3a was formed via 4 considering the fact that the present transformation is a two step process, i.e. the reaction of 1a with 2a to give 4 which on intramolecular cyclization affords 3a. It was therefore clear that the conversion of 4 to 3a was not efficient under these conditions and perhaps a stronger acidic condition was necessary for this intramolecular cyclization. Moreover, the higher yield of 3a was obtained in the presence of AcOH (in toluene) (entry 8 vs. 1–7, Table 1) among all the catalysts used. Thus the reaction of 1a with 2a was performed in AcOH alone that played the role of catalyst as well as solvent. To our delight, the reaction proceeded well affording the desired product 3a in 89% yield (entry 9, Table 1). The compound 4 was not isolated even in trace quantity in this case. To assess the role of AcOH the reaction was performed in absence of it (under neat condition) when 4 was isolated in 80% yield (entry 10, Table 1). It was therefore evident that the reaction of 1a with 3a leading to 4 does not require any catalyst whereas conversion of 4 to 3a is facilitated by an acid catalyst. This was further supported by the fact that compound 3a was obtained in good yield when the compound 4 was stirred in AcOH under reflux for 4 h. Nevertheless, the condition of entry 9 of Table 1 was identified as the best one for the preparation of 3a and its analogues.
Table 1 Reaction of 1a with 2a under various conditionsa
|
| Entry |
Catalyst (10%)/solvent |
Time (h) |
Yieldb (%) |
| 3a |
4 |
| Reaction conditions: compound 1a (1.0 mmol), amine 2a (1.1 mmol) and catalyst (10% w/w) in a solvent (10 mL) at refluxing temperature (80–120 °C) under open air. Isolated yield. No additional catalyst was used. No additional solvent was used. |
| 1 |
H2SO4/EtOH |
8 |
5 |
40 |
| 2 |
TFA/EtOH |
8 |
10 |
50 |
| 3 |
PTSA/EtOH |
8 |
10 |
45 |
| 4 |
AcOH/EtOH |
8 |
10 |
55 |
| 5 |
H2SO4/toluene |
8 |
20 |
65 |
| 6 |
TFA/toluene |
8 |
25 |
70 |
| 7 |
PTSA/toluene |
8 |
23 |
68 |
| 8 |
AcOH/toluene |
8 |
30 |
70 |
| 9 |
AcOH |
6.5 |
89c |
0 |
| 10 |
Neatd |
8 |
10 |
80 |
To expand the generality and scope of this method the optimized reaction conditions were used to perform the reaction of a range of chloro compounds5,6 (1) with aromatic 1,2-diamines (2) (Table 2). The chloro compound (1) may contain various groups at N-1 including Me, n-propyl, 1-propenyl, 1-butenyl, n-hexyl, benzyl or alkylaryl (entries 2–8 and 10–13, Table 2). The reactions were completed within 5.5–6.5 h affording the corresponding indolofuroquinoxaline derivatives (3a–m) in good yields. Notably, these reactions did not require the use of inert or anhydrous atmosphere as the processes were found to be not sensitive towards aerial oxygen or moisture. Moreover, MeOH, H2O and HCl (that can be neutralized by NaOH to harmless NaCl) being the byproducts in these reactions all the products were isolated in pure form after treating with water followed by trituration with methyl t-butyl ether (MTBE) (see the Experimental section). The scale-up potential of this method was also examined by performing the reaction of 1a with 2a (cf. entry 9, Table 1) in g scale [i.e. 5 g (21.04 mmol) of 1a and 2.5 g (23.14 mmol) of 2a] when 3a was isolated in 92% yield.
Table 2 Synthesis of 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives (3)a
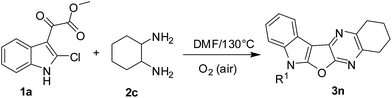
Having prepared a variety of indolofuroquinoxaline derivatives (3a–m) using aromatic 1,2-diamines (2a and b) we focused on the use of aliphatic 1,2-diamines in the present reaction. Interestingly, it was observed that the acidic condition was not favorable for the formation of expected product when an aliphatic (±)-trans-1,2-diaminocyclohexane (2c) was reacted with 1a (entries 1–4, Table 3) perhaps due to the quick protonation of 2c thereby preventing its reaction with 1a. However, the reaction proceeded well when performed under neutral condition in a solvent in the absence of any catalyst (entries 5–6, Table 3). Among the three solvents examined (e.g. toluene, o-xylene and DMF) (entries 5–7, Table 3) DMF was found to be marginally better than others in terms of product yield (entry 7, Table 3). Notably, no uncyclized intermediate (cf. 4, Table 1) was obtained as byproduct in these cases. Unlike the reactions of Table 2, the aerial oxygen seemed to have a role in the present case that perhaps facilitated the oxidation of the fused C–C bond of the cyclohexane ring (see the reaction mechanism). Nevertheless, the condition of entry 7 of Table 3 was used for the preparation of desired compounds using aliphatic 1,2-diamine (Scheme 3). An attempt to perform the reaction of 1a with 2a under the condition of entry 7 of Table 3 afforded compound 4 in 70% yield instead of 3a. This observation once again confirmed the need of an acid catalyst during the conversion of 4 to 3a. We also examined the use of starting chloro compound (1c–e) having a substituent at C-5 of the indole ring (Scheme 4). The reaction proceeded well in these cases affording the desired products 3p–r in good yields.
Table 3 Study of reaction of 1a with 2ca
|
| Entry |
Catalyst (10%)/solvent |
Temp (°C) |
Time (h) |
Yieldb (%) |
| Reaction conditions: chloro compound 1a (1.0 mmol), amine 2c (1.1 mmol) and catalyst (10% w/w) in a solvent (10 mL) under open air. Isolated yield. No catalyst was used. |
| 1 |
H2SO4/EtOH |
80 |
8 |
0 |
| 2 |
TFA/EtOH |
80 |
8 |
0 |
| 3 |
AcOH/EtOH |
80 |
8 |
0 |
| 4 |
AcOH |
120 |
6.5 |
0 |
| 5 |
Toluene |
110 |
24 |
52c |
| 6 |
o-Xylene |
130 |
24 |
55c |
| 7 |
DMF |
130 |
18 |
65c |
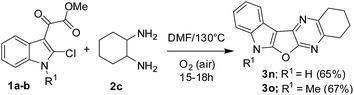 |
| | Scheme 3 Synthesis of 2,3,4,7-tetrahydro-1H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline and its analogue (3n–o). | |
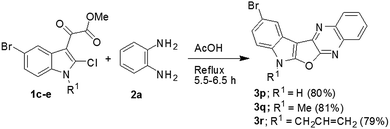 |
| | Scheme 4 Synthesis of 10-bromo-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline and its analogue (3p–r). | |
Based on the results of Tables 1 and 3 a plausible reaction mechanism is depicted in Scheme 5. The reaction seemed to proceed via formation of 1,4-quinoxalin-2-one based intermediate7 (E-1) in situ that may tautomerizes to E-2. Activation of C-2 chloro substituent of the indole ring of E-2 via H-bonding involving N-4 of 1,4-quinoxalin ring with HZ (Z = OH, OMe or OAc, depending on the reaction conditions employed) allows intramolecular ring closure of E-3 involving a nucleophilic attack by the proximate OH group on the C-2 of indole ring that followed an addition–elimination mechanism.8 Thus the resultant intermediate E-4 then affords the desired product 3 after releasing HCl. In case of reaction of 1 with 1,2-diamine 2c the reaction proceeds through an additional intermediate E to form the E-1 like intermediate (Scheme 6). Notably, the displacement of chloro group from N-unsubstituted 2-chloro-1H-indole-3-carbaldehyde by a nucleophile was found to be unsatisfactory earlier.8b However, the reaction proceeded well irrespective of presence or absence of a substituent on indole nitrogen of 1 in the present case.
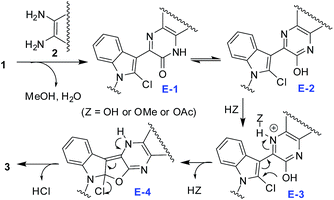 |
| | Scheme 5 Proposed reaction mechanism. | |
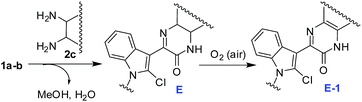 |
| | Scheme 6 Proposed reaction mechanism for the reaction of 1a and b with 2c. | |
Conclusions
In conclusion, we have demonstrated that the 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives can be synthesized directly from methyl-2-(2-chloro-1H-indol-3-yl)-2-oxoacetate or its N-alkyl derivatives via a cascade reaction under neutral or mild acidic conditions. This one-pot methodology has remarkable advantages over the previously reported multi-step method as it avoids the use of environmentally harmful POCl3 and strong alkali required for the earlier method. It is also amenable for scale-up. The majority of compounds synthesized is novel and not studied earlier. The present method therefore provides an easy and rare access to 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives for further pharmacological studies. The methodology therefore may find usage in building library of small molecules related to this class of heterocycles.
Experimental
General methods
All reagents were used as received from commercial sources without further purification or prepared as described in the literature. Reactions were stirred using Teflon-coated magnetic stirring bars. TLC plates were visualized by ultraviolet light or by treatment with a spray of Pancaldi reagent {(NH4)6MoO4, Ce(SO4)2, H2SO4, H2O}. Chromatographic purification of products was carried out by flash column chromatography on silica gel (60–120 mesh). Melting points were determined using an electro thermal melting point apparatus and are uncorrected. NMR spectra were recorded in CDCl3 or DMSO-d6 (all with TMS as internal standard) on a Varian Gemini 400 MHz FT NMR spectrometer. Chemical shifts (δ) are reported in ppm, and coupling constants (J) are in Hz. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. Mass spectra were recorded on an HP-5989A quadrapole mass spectrometer.
Preparation of methyl-2-(2-chloro-1H-indol-3-yl)-2-oxoacetate (1a)4
To a vigorously stirred solution of oxalyl chloride (122 mL, 1.4 mol, 2 equiv.) in 500 mL of dichloromethane was added oxindole (93 g, 0.7 mol, 1 equiv.) in portions at room temperature and stirring continued for 20 h at room temperature. The formed beige slurry was filtered, washed with dichloromethane (4 × 100 mL) and dried in vacuum. The solid was re-suspended in diethyl ether (400 mL) to which methanol (46 mL, 1.1 mol, 2 equiv.) was added at room temperature. After a few seconds a beige precipitate appeared and the mixture was stirred for additional 15 min. The precipitate was filtered, carefully washed with diethyl ether (3 × 250 mL) and dried under vacuum to afford the desired compound as a beige solid (140 g, 85% yield).1H NMR (400 MHz, DMSO-d6): δ 13.44 (s, br, 1H, NH), 8.04 (dd, J1 = 6.8 Hz, J1 = 2.0 Hz, 1H, ArH), 7.46 (dd, J1 = 7.2 Hz, J1 = 1.6 Hz, 1H, ArH), 7.33–7.26 (m, 2H, ArH), 3.91 (s, 3H); 13C NMR (100 MHz, DMSO-d6): δ 180.7, 165.2, 134.7, 133.4, 125.4, 124.3, 123.4, 120.2, 111.9, 107.5, 52.8; HRMS: m/z [M + 1] calcd for C11H9NO3Cl (M + H): 238.0271; found: 238.0260.
General procedure for the preparation of N-substituted-2-(2-chloro-1H-indol-3-yl)-2-oxoacetate (1b–i)
To a suspension of methyl-2-(2-chloro-1H-indol-3-yl)-2-oxoacetate (1a, 1 mmol) in DMF (10 mL) was added sodium hydride (1.05 mmol) followed by an appropriate alkyl halide (1.05 mmol) at 0 ± 5 °C. The mixture was stirred at 0 ± 5 °C for 1 h. The reaction was monitored by TLC. Upon completion of the reaction, the mixture was diluted with water (10 mL) and the solid appeared was filtered under vacuum. The solid obtained was dried to give the desired product.
Methyl-2-(2-chloro-1-methyl-1H-indol-3-yl)-2-oxoacetate (1b). Brown solid; yield: 95%; mp: 100–102 °C; 1H NMR (400 MHz, CDCl3): δ 8.28–8.26 (m, 1H, ArH), 7.36–7.31 (m, 3H, ArH), 3.98 (s, 3H), 3.81 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 180.7, 165.4, 135.9, 134.6, 125.4, 124.4, 123.9, 121.6, 109.7, 108.9, 52.7, 30.5; HRMS: m/z [M + 1] calcd for C12H11NO3Cl (M + H): 252.0427; found: 252.0422.
Methyl-2-(2-chloro-1-propyl-1H-indol-3-yl)-2-oxoacetate (1c). White solid; yield: 94%; mp: 90–92 °C; 1H NMR (400 MHz, CDCl3): δ 8.29–8.27 (m, 1H, ArH), 7.34–7.32 (m, 3H, ArH), 4.23 (t, J = 7.4 Hz, 2H), 3.98 (s, 3H), 1.90–184 (q, J = 7.3 Hz, 2H), 1.01 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 180.8, 165.5, 135.3, 134.1, 125.6, 124.3, 123.8, 121.7, 109.9, 108.8, 52.7, 45.8, 22.6, 11.3; HRMS: m/z [M + 1] calcd for C14H15NO3Cl (M + H): 280.0740; found: 280.0758.
Methyl-2-(1-allyl-2-chloro-1H-indol-3-yl)-2-oxoacetate (1d). White solid; yield: 93%; mp: 68–70 °C; 1H NMR (400 MHz, CDCl3): δ 8.30–8.28 (m, 1H, ArH), 7.36–7.26 (m, 3H, ArH), 5.97–5.88 (m, 1H), 5.28 (d, J = 10.4 Hz, 1H), 5.07 (d, J = 17.2 Hz, 1H), 4.88–4.86 (m, 2H), 3.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 180.8, 165.3, 135.3, 134.1, 130.3, 125.5, 124.4, 123.9, 121.7, 118.4, 110.0 (2C), 52.7, 46.2; HRMS: m/z [M + 1] calcd for C14H13NO3Cl (M + H): 278.0584; found: 278.0596.
Methyl-2-(1-(but-3-en-1-yl)-2-chloro-1H-indol-3-yl)-2-oxoacetate (1e). White solid; yield: 91%; mp: 105–107 °C; 1H NMR (400 MHz, CDCl3): δ 8.29–8.26 (m, 1H, ArH), 7.35–7.26 (m, 3H, ArH), 5.84–5.74 (m, 1H), 5.10–5.06 (m, 2H), 4.23 (t, J = 7.2 Hz, 2H), 3.97 (s, 3H), 2.59–2.54 (q, J = 7.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 180.8, 165.4, 135.2, 133.9, 132.9, 125.6, 124.3, 123.8, 121.7, 118.6, 109.9, 108.9, 52.6, 43.7, 33.4; HRMS: m/z [M + 1] calcd for C15H15NO3Cl (M + H): 292.0740; found: 292.0736.
Methyl-2-(2-chloro-1-hexyl-1H-indol-3-yl)-2-oxoacetate (1f). White solid; yield: 93%; mp: 108–110 °C; 1H NMR (400 MHz, CDCl3): δ 8.29–8.27 (m, 1H, ArH), 7.34–7.26 (m, 3H, ArH), 4.25 (t, J = 7.6 Hz, 2H), 3.98 (s, 3H), 1.85–1.78 (m, 2H), 1.40–1.26 (m, 6H), 0.9 (t, J = 6.8 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 180.7, 165.4, 135.3, 133.9, 125.6, 124.2, 123.8, 121.7, 109.8, 108.8, 52.6, 44.3, 31.3, 29.2, 26.4, 22.4, 13.9; HRMS: m/z [M + 1] calcd for C17H21NO3Cl (M + H): 322.1210; found: 322.1213.
Methyl-2-(1-benzyl-2-chloro-1H-indol-3-yl)-2-oxoacetate (1g). Light brown color solid; yield: 93%; mp: 40–42 °C; 1H NMR (400 MHz, CDCl3): δ 8.31 (d, J = 7.2 Hz, 1H, ArH), 7.36–7.26 (m, 6H, ArH), 7.13 (d, J = 6.4 Hz, 2H, ArH), 5.46 (s, 2H), 3.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 180.9, 165.3, 135.6, 134.7, 129.1 (3C), 128.2, 126.5 (3C), 125.6, 124.6, 124.0, 121.7, 110.3, 52.7, 47.6; HRMS: m/z [M + 1] calcd for C18H15NO3Cl (M + H): 328.0740; found: 328.0750.
Methyl-2-(2-chloro-1-(2-fluorobenzyl)-1H-indol-3-yl)-2-oxoacetate (1h). White solid; yield: 92%; mp: 132–134 °C; 1H NMR (400 MHz, CDCl3): δ 8.31 (t, J = 6.8 Hz, 1H, ArH), 7.37–7.26 (m, 3H, ArH), 7.15 (t, J = 9.0 Hz, 1H, ArH), 7.05 (t, J = 7.6 Hz, 1H, ArH), 6.81 (t, J = 7.0 Hz, 1H, ArH), 5.51 (s, 2H), 3.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 180.9, 165.3, 161.2 (d, J = 247.6 Hz), 135.5, 134.3, 130.1 (d, J = 8.45 Hz), 128.0 (d, J = 3.20 Hz), 125.5, 124.8, 124.7 (2C), 124.1 (2C), 121.9 (d, J = 14.59 Hz), 115.8 (d, J = 20.74 Hz), 110.0 (d, J = 59.98 Hz), 52.8, 41.3 (d, J = 5.33 Hz); HRMS: m/z [M + 1] calcd for C18H14NO3ClF (M + H): 346.0646; found: 346.0650.
Methyl-2-(2-chloro-1-(2-chlorobenzyl)-1H-indol-3-yl)-2-oxoacetate (1i). White solid; yield: 92%; mp: 137–138 °C; 1H NMR (400 MHz, CDCl3): δ 8.34 (d, J = 7.6 Hz, 1H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.38–7.19 (m, 4H, ArH), 7.13 (t, J = 7.2 Hz, 1H, ArH), 6.48 (d, J = 8.0 Hz, 1H, ArH), 5.56 (s, 2H), 3.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 180.9, 165.2, 135.5, 134.5, 132.1, 132.0, 129.8, 129.3, 127.5, 126.7, 125.6, 124.8, 124.2, 121.8, 110.1, 109.5, 52.8, 45.1; HRMS: m/z [M + 1] calcd for C18H14NO3Cl2 (M + H): 362.0351; found: 362.0361.
General procedure for the preparation of compound 3a–m and 3p–r
A suspension of chloro-ester 1 (1.0 mmol), and amine (2, 1.1 mmol) in acetic acid (10 mL) was heated to reflux (the mixture became a clear solution as soon as the temperature reached to 50 °C) and stirred for the time mentioned in Table 2. The reaction was monitored by TLC. The product appeared as a fluorescent spot in the TLC. Upon completion of the reaction the mixture was cooled to room temperature and diluted with water (10 mL). The solid precipitated was filtered and dried under reduced pressure. The solid obtained was titurated in MTBE (10 mL), filtered and dried under vacuum to give the analytically pure desired product.
Scale-up of compound 3a
A suspension of chloro-ester 1a (5 g, 21.04 mmol), and amine 2a (2.5 g, 23.14 mmol) in acetic acid (50 mL) was heated to reflux and stirred for 6.5 h. The reaction was monitored by TLC. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with water (50 mL). The solid precipitate was filtered and dried under reduced pressure. The solid obtained was titurated in MTBE (50 mL), filtered and dried under vacuum to give the desired product 3a (5.0 g, yield: 92%).
General procedure for the preparation of compound 3n and o
A solution of chloro-ester 1 (1.0 mmol), and aliphatic amine (2, 1.1 mmol) in DMF (10 mL) was stirred at 130 °C for 15–18 h. The reaction was monitored by TLC. The product appeared as a fluorescent spot in the TLC. Upon completion of the reaction, the mixture was cooled to room temperature and diluted with water (10 mL). The solid precipitate was filtered and dried under reduced pressure. The solid obtained was titurated in MTBE (10 mL), filtered and dried under vacuum to give the desired product.
7H-Indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3a). Yellow solid; yield: 89%; mp: 279–281 °C; 1H NMR (400 MHz, DMSO-d6): δ 13.21 (s, br, 1H, NH), 8.16 (d, J = 8.4 Hz, 1H, ArH), 8.05 (d, J = 8.0 Hz, 1H, ArH), 7.94 (dd, J = 6.8 Hz, J = 3.6 Hz, 1H, ArH), 7.82 (t, J = 7.6 Hz, 1H, ArH), 7.73 (t, J = 7.6 Hz, 1H, ArH), 7.62–7.60 (m, 1H, ArH), 7.35–7.33 (m, 2H, ArH); 13C NMR (100 MHz, DMSO-d6): δ 161.5, 157.0, 140.9, 139.5, 136.8, 135.5, 128.3, 128.1, 127.6, 126.7, 122.7, 122.1, 119.8, 119.5, 113.4, 95.1; HRMS: m/z [M + 1] calcd for C16H10N3O (M + H): 260.0824; found: 260.0832.
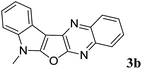
7-Methyl-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3b). Yellow solid; yield: 87%; mp: 175–177 °C; 1H NMR (400 MHz, CDCl3): δ 8.19 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, ArH), 8.13 (dd, J = 6.4 Hz, J = 1.2 Hz, 1H, ArH), 8.05 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, ArH), 7.75–7.71 (m, 1H, ArH), 7.66–7.62 (m, 1H, ArH), 7.41–7.26 (m, 3H, ArH), 3.94 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 161.0, 157.0, 141.5, 139.8, 137.8, 135.9, 128.4, 128.3, 127.9, 126.7, 122.6, 122.5, 120.8, 119.9, 110.2, 95.3, 29.3; HRMS: m/z [M + 1] calcd for C17H12N3O (M + H): 274.0980; found: 274.0991.
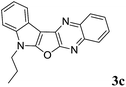
7-Propyl-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3c). Orange solid; yield: 85%; mp: 157–159 °C; 1H NMR (400 MHz, CDCl3): δ 8.21–8.15 (m, 2H, ArH), 8.06 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, ArH), 7.76–7.72 (m, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.47 (dd, J = 6.8 Hz, J = 2.4 Hz, 1H, ArH), 7.41–7.26 (m, 2H, ArH), 4.35 (t, J = 7.0 Hz, 2H), 2.09–2.03 (m, 2H), 1.06 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 161.2, 157.2, 141.5, 139.9, 137.3, 135.9, 128.4 (2C), 127.8, 126.8, 122.6, 122.4, 121.0, 120.1, 110.6, 95.3, 45.3, 22.5, 11.4; HRMS: m/z [M + 1] calcd for C19H16N3O (M + H): 302.1293; found: 302.1288.
7-Allyl-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3d). Yellow color solid; yield: 84%; mp: 175–177 °C; 1H NMR (400 MHz, CDCl3): δ 8.22 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, ArH), 8.20 (dd, J = 11.6 Hz, J = 1.2 Hz, 1H, ArH), 8.07 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, ArH), 7.77–7.73 (m, 1H, ArH), 7.68–7.64 (m, 1H, ArH), 7.46–7.26 (m, 3H, ArH), 6.15–6.05 (m, 1H), 5.36–5.29 (m, 2H), 4.99 (d, J = 6.0 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 160.9, 157.2, 141.7, 139.9, 137.3, 135.1, 130.9, 128.5, 128.4, 127.9, 126.9, 122.8, 122.6, 121.0, 120.3, 119.0, 110.9, 95.7, 45.9; HRMS: m/z [M + 1] calcd for C19H14N3O (M + H): 300.1137; found: 300.1133.
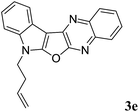
7-(But-3-en-1-yl)-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3e). Yellow color solid; yield: 82%; mp: 165–167 °C; 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 8.4 Hz, J = 3.2 Hz, 1H, ArH), 8.17–8.12 (m, 1H, ArH), 8.07 (dd, J = 8.0 Hz, J = 1.2 Hz, 1H, ArH), 7.76–7.72 (m, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.41–7.26 (m, 2H, ArH), 5.91–5.81 (m, 2H), 5.10–5.04 (m, 2H), 4.44 (t, J = 7.2 Hz, 2H), 2.79 (q, J = 6.8 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 161.1, 157.2, 141.6, 139.9, 137.1, 136.0, 133.3, 128.5, 128.4, 127.9, 126.9, 122.7, 122.5, 121.1, 120.2, 118.7, 110.6, 95.5, 43.2, 33.4; HRMS: m/z [M + 1] calcd for C20H16N3O (M + H): 314.1293; found: 314.2299.
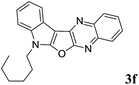
7-Hexyl-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3f). Orange solid; yield: 83%; mp: 110–112 °C; 1H NMR (400 MHz, CDCl3): δ 8.21 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, ArH), 8.16 (dd, J = 6.0 Hz, J = 2.0 Hz, 1H, ArH), 8.07 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, ArH), 7.76–7.72 (m, 1H, ArH), 7.67–7.63 (m, 1H, ArH), 7.47 (dd, J = 6.8 Hz, J = 3.2 Hz, 1H, ArH), 7.41–7.26 (m, 2H, ArH), 4.38 (t, J = 7.0 Hz, 2H), 2.05–1.98 (m, 2H), 1.62–1.25 (m, 6H), 0.89 (t, J = 7.2 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 161.1, 157.3, 141.6, 139.9, 137.3, 136.0, 128.5, 128.4, 127.9, 126.8, 122.6, 122.4, 121.0, 120.2, 110.6, 95.4, 43.8, 31.2, 29.1, 26.5, 22.4, 13.9; HRMS: m/z [M + 1] calcd for C22H22N3O (M + H): 344.1763; found: 344.1772.
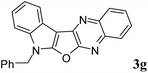
7-Benzyl-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3g). Yellow solid; yield: 85%; mp: 185–187 °C; 1H NMR (400 MHz, CDCl3): δ 8.2 (dd, J = 8.4 Hz, J = 1.2 Hz, 1H, ArH), 8.16 (d, J = 9.0 Hz, 1H, ArH), 8.07 (dd, J = 8.4 Hz, J = 0.8 Hz, 1H, ArH), 7.77–7.73 (m, 1H, ArH), 7.68–7.64 (m, 1H, ArH), 7.44 (d, J = 7.2 Hz, 1H, ArH), 7.40–7.26 (m, 7H, ArH), 5.55 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 160.9, 157.2, 141.7, 139.9, 137.3, 136.1, 134.9, 129.1 (2C), 128.5, 128.5, 128.5, 128.0, 127.3 (2C), 126.9, 122.9, 122.7, 121.1, 120.4, 111.1, 95.9, 47.3; HRMS: m/z [M + 1] calcd for C23H16N3O (M + H): 350.1293; found: 350.1282.
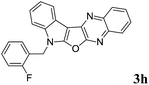
7-(2-Fluorobenzyl)-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3h). Yellow solid; yield: 84%; mp: 175–177 °C; 1H NMR (400 MHz, CDCl3): δ 8.22–8.15 (m, 2H, ArH), 8.08 (dd, J = 8.4 Hz, J = 0.8 Hz, 1H, ArH), 7.77–7.73 (m, 1H, ArH), 7.68–7.64 (m, 1H, ArH), 7.51 (d, J = 8.0 Hz, 1H, ArH), 7.41–7.26 (m, 4H, ArH), 7.15–7.09 (m, 2H, ArH), 5.60 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 161.7 (d, J = 78.40 Hz), 159.2 (d, J = 206.03 Hz), 141.6, 139.9, 137.2, 136.1, 130.5 (d, J = 8.45 Hz), 129.7, 129.6, 128.5, 127.9, 127.0, 124.8 (d, J = 3.82 Hz), 122.9 (d, J = 21.43 Hz), 122.1, 121.9, 121.0, 120.2, 115.9 (d, J = 21.54 Hz), 110.9, 110.9, 95.9, 40.9 (d, J = 4.63 Hz); HRMS: m/z [M + 1] calcd for C23H15N3OF (M + H): 368.11199; found: 368.1198.
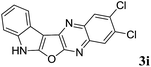
2,3-Dichloro-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3i). Brown color solid; yield: 80%; mp: 230–232 °C; 1H NMR (400 MHz, DMSO-d6): δ 8.15 (s, 1H), 8.09 (s, 1H, ArH), 7.73 (t, J = 3.4 Hz, 1H, ArH), 7.48 (d, J = 4.8 Hz, 1H, ArH), 7.14 (d, J = 2.8 Hz, 2H, ArH); 13C NMR (100 MHz, DMSO-d6): δ 171.5, 159.9, 147.7, 141.1, 140.5, 134.4, 129.2, 128.1, 126.7, 125.0, 123.1, 120.8, 119.6, 118.6, 116.9, 93.6; HRMS: m/z [M + 1] calcd for C16H8N3OCl2 (M + H): 328.0044; found: 328.0037.
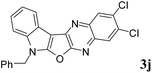
7-Benzyl-2,3-dichloro-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3j). Yellow solid; yield: 80%; mp: 183–185 °C; 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H, ArH), 8.12 (s, 1H, ArH), 8.11 (d, J = 8.0 Hz, 1H, ArH), 7.44–7.26 (m, 8H, ArH), 5.53 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 161.4, 157.7, 140.6, 140.4, 137.5, 134.9, 134.7, 132.7, 130.7, 129.2 (2C), 128.9, 128.5 (2C), 127.3 (2C), 123.3, 123.0, 121.1, 120.1, 111.2, 95.9, 47.4; HRMS: m/z [M + 1] calcd for C23H14N3OCl2 (M + H): 418.0514; found: 418.0543.
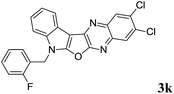
2,3-Dichloro-7-(2-fluorobenzyl)-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3k). Greenish yellow solid; yield: 79%; mp: 201–203 °C; 1H NMR (400 MHz, CDCl3): δ 8.27 (s, 1H, ArH), 8.12 (s, 1H, ArH), 8.10 (dd, J1 = 8.4 Hz, J2 = 1.2 Hz, 1H, ArH), 7.50 (d, J = 8.0 Hz, 1H, ArH), 7.41–7.26 (m, 4H, ArH), 7.15–7.08 (m, 2H, ArH), 5.58 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 161.7 (d, J = 31.50 Hz), 159.3 (d, J = 161.04 Hz), 140.5, 140.4, 137.3, 134.9, 132.7, 130.8, 130.6 (d, J = 7.05 Hz), 129.7, 129.6, 129.0, 128.6, 124.8 (d, J = 3.82 Hz), 123.4, 123.1, 121.9 (d, J = 14.56 Hz), 121.1, 120.0, 116.1 (d, J = 20.73 Hz), 111.1, 95.9, 41.1 (d, J = 4.63 Hz); HRMS: m/z [M + 1] calcd for C23H13N3OFCl2 (M + H): 436.0420; found: 436.0447.
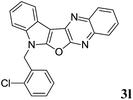
7-(2-Chlorobenzyl)-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3l). Yellow solid; yield: 80%; mp: 196–197 °C; 1H NMR (400 MHz, CDCl3): δ 8.23–8.17 (m, 2H, ArH), 8.07 (dd, J = 8.4 Hz, J = 0.8 Hz, 1H, ArH), 7.77–7.60 (m, 2H, ArH), 7.47 (d, J = 8.0 Hz, 1H, ArH), 7.42–7.22 (m, 4H, ArH), 7.19 (t, J = 7.2 Hz, 1H, ArH), 7.02 (d, J = 8.0 Hz, 1H, ArH), 5.67 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 161.1, 157.2, 141.6, 139.9, 137.3, 136.2, 132.9, 132.4, 129.9, 129.6, 128.5 (2C), 128.4, 128.0, 127.4, 127.0, 123.0, 122.8, 121.1, 120.3, 111.1, 96.0, 44.9; HRMS: m/z [M + 1] calcd for C23H15N3OCl (M + H): 384.0904; found: 384.0911.
2,3-Dichloro-7-(2-chlorobenzyl)-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3m). Yellow color solid; yield: 79%; mp: 246–247 °C; 1H NMR (400 MHz, CDCl3): δ 8.28 (s, 1H, ArH), 8.14 (s, 1H, ArH), 8.12 (s, 1H, ArH), 7.43–7.26 (m, 5H, ArH), 7.20 (t, J = 7.6 Hz, 1H, ArH), 7.02 (d, J = 8.0 Hz, 1H, ArH), 5.65 (s, 2H); 13C NMR (100 MHz, CDCl3): δ 161.9, 157.6, 140.6, 140.4, 137.5, 135.0, 132.9, 132.8, 132.1, 130.8, 130.0, 129.8, 129.0, 128.6, 128.4, 127.5, 123.5, 123.2, 121.2, 120.1, 111.3, 95.1, 45.0; HRMS: m/z [M + 1] calcd for C23H13N3OCl3 (M + H): 452.0124; found: 452.0124.
2,3,4,7-Tetrahydro-1H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3n). Yellow color solid; yield: 65%; mp: 230–232 °C; 1H NMR (400 MHz, DMSO-d6): δ 12.78 (s, br, 1H, NH), 7.83–7.81 (m, 1H, ArH), 7.56–7.53 (m, 1H, ArH), 7.28–7.25 (m, 2H, ArH), 2.99 (d, J = 5.2 Hz, 2H), 2.91 (d, J = 7.2 Hz, 2H), 1.90 (t, J = 3.2 Hz, 4H); 13C NMR (100 MHz, DMSO-d6): δ 157.9, 155.0, 147.1, 139.9, 136.4, 135.5, 122.0, 121.3, 119.5, 119.2, 112.9, 95.3, 31.3, 31.1, 22.4, 22.3; HRMS: m/z[M + 1] calcd for C16H14N3O (M + H): 264.1137; found: 264.1126.
7-Methyl-2,3,4,7-tetrahydro-1H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3o). Yellow color solid; yield: 67%; mp: 200–201 °C; 1H NMR (400 MHz, CDCl3): δ 8.10 (t, J = 4.4 Hz, 1H, ArH), 7.43 (t, J = 4.8 Hz, 1H, ArH), 7.36–7.26 (m, 2H, ArH), 3.93 (s, 3H), 3.11–3.01 (m, 4H), 1.98 (d, J = 3.6 Hz, 4H); 13C NMR (100 MHz, CDCl3): δ 147.4, 140.5, 122.2 (3C), 121.8 (3C), 120.9 (2C), 119.9, 110.0, 31.9, 31.7, 29.2, 22.9, 22.8; HRMS: m/z[M + 1] calcd for C17H16N3O (M + H): 278.1293; found: 278.1298.
10-Bromo-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3p). Yellow solid; yield: 80%; mp: 295–297 °C; 1H NMR (400 MHz, DMSO-d6): δ 12.70 (s, br, 1H, NH), 8.15 (d, J = 8.4 Hz, 1H, ArH), 8.05 (d, J = 8.0 Hz, 2H, ArH), 7.82 (t, J = 7.2 Hz, 1H, ArH), 7.74 (t, J = 7.6 Hz, 1H, ArH), 7.58 (d, J = 8.8 Hz, 1H, ArH), 7.47 (d, J = 8.4 Hz, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ 161.0, 158.3, 140.7, 140.2, 137.7, 129.4, 128.2, 128.1, 127.4, 123.0, 122.1, 121.5, 119.1, 112.7, 111.8, 93.2; HRMS: m/z[M + 1] calcd forC16H9N3OBr (M + H): 337.9929; found: 337.9932.
10-Bromo-7-methyl-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3q). Yellow solid; yield: 81%; mp: 265–267 °C; 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 1.6 Hz, 1H, ArH), 8.13 (d, J = 8.0 Hz, 1H, ArH), 7.99 (d, J = 8.2 Hz, 1H, ArH), 7.70 (t, J = 7.4 Hz, 1H, ArH), 7.61 (t, J = 7.4 Hz, 1H, ArH), 7.41 (dd, J = 8.8 Hz, J = 1.2 Hz, 1H, ArH), 7.24 (d, J = 8.8 Hz, 1H, ArH), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 161.2, 157.0, 141.6, 139.6, 136.6, 136.2, 128.6 (2C), 128.5, 128.1, 127.2, 125.5, 123.6, 121.5, 116.0, 111.7, 29.3; HRMS: m/z [M + 1] calcd for C17H11N3OBr (M + H): 352.0104; found: 352.0085.
7-Allyl-10-bromo-7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline (3r). Orange color solid; yield: 79.0%; mp: 190–192 °C; 1H NMR (400 MHz, CDCl3): δ 8.24 (s, 1H, ArH), 8.14 (d, J = 8.0 Hz, 1H, ArH), 8.0 (d, J = 8.0 Hz, 1H, ArH), 7.71 (t, J = 7.6 Hz, 1H, ArH), 7.62 (t, J = 7.6 Hz, 1H, ArH), 7.39 (d, J = 8.8 Hz, 1H, ArH), 7.25 (d, J = 8.8 Hz, 1H, ArH), 6.04–5.97 (m, 1H), 5.30–5.12 (m, 2H), 4.90 (d, J = 5.2 Hz, 2H); 13C NMR (100 MHz, CDCl3): δ 160.9, 157.2, 141.7, 136.3, 135.9, 130.5, 128.7, 128.6, 128.1, 127.3, 125.6, 123.8, 121.7, 119.3, 116.0, 112.4, 110.0, 95.7, 46.1; HRMS: m/z [M + 1] calcd for C19H13N3OBr (M + H): 378.0226; found: 378.0242.
3-(2-Chloro-1H-indol-3-yl)quinoxalin-2-(1H)-one (4)
Yellow solid; yield: 85%; mp: 256–258 °C; 1H NMR (400 MHz, DMSO-d6): δ 12.49 (s, br, 1H, NH), 12.37 (s, br, 1H, NH), 7.81 (d, J = 8.4 Hz, 1H, ArH), 7.68 (d, J = 8.4 Hz, 1H, ArH), 7.56 (t, J = 7.6 Hz, 1H, ArH), 7.39–7.31 (m, 3H, ArH), 7.21 (t, J = 7.6 Hz, 1H, ArH), 7.12 (d, J = 7.2 Hz, 1H, ArH); 13C NMR (100 MHz, DMSO-d6): δ 153.9, 152.5, 134.3, 132.2, 131.7, 129.8, 128.4, 126.8, 125.0, 123.3, 122.2, 120.5, 120.3, 115.1, 110.9, 108.7; HRMS: m/z [M + 1] calcd for C16H11N3OCl (M + H): 296.0591; found: 296.0560.
Acknowledgements
S. P. N. thanks to Dr Vilas Dahanukar, Dr Rama Mohan, Dr K. B. Shiva Kumar and the analytical group of CPS-DRL for support.
Notes and references
-
(a) J. Harmenberg, A. Gr, T. Malmfors, J. Bergman, B. Wahren and S. Cox, Antiviral Res., 1991, 15, 193 CrossRef CAS PubMed;
(b) K. Hirata, J. Araya, S. Nakaike, K. Kitamura and T. Ishida, Chem. Pharm. Bull., 2001, 49, 44 CrossRef CAS PubMed;
(c) S. T. Hazeldine, L. Polin, J. Kushner, K. White, T. H. Corbett and J. P. Horwitz, Bioorg. Med. Chem., 2006, 14, 2462 CrossRef CAS PubMed;
(d) For a review, see: N. S. Moorthy, E. Manivannan, C. Karthikeyan and P. Trivedi, Mini-Rev. Med. Chem., 2013, 13, 1415 CrossRef PubMed.
- S. P. Nikumbh, A. Raghunadh, V. N. Murthy, R. Jinkala, S. C. Joseph, Y. L. N. Murthy, B. Prasad and M. Pal, RSC Adv., 2015, 5, 74570 RSC.
- M. Stiborová, J. Poljaková, E. Martínková, L. Bořek-Dohalská, T. Eckschlager, R. Kizek and E. Frei, Interdiscip. Toxicol., 2011, 4, 98, DOI:10.2478/v10102-011-0017-7.
- W. Fröhner, B. Monse, T. M. Braxmeier, L. Casiraghi, H. Sahagún and P. Seneci, Org. Lett., 2005, 7, 4573 CrossRef PubMed.
- J. R. De la Fuente, A. Cañete, A. L. Zanocco, C. Saitz and C. Jullian, J. Org. Chem., 2000, 65, 7949 CrossRef CAS PubMed.
- All the N-substituted chloro compounds (1b–i) were conveniently prepared via the reaction of 1a with appropriate alkyl halide in the presence of NaH in DMF.
- For condensation of 1,2-diamine with 1,2-dicarbonyl derivatives leading to quinoxalines, see:
(a) A. E. A. Porter, in “Comprehensive Heterocyclic Chemistry”, ed. A. R. Katritzky and C. W. Rees, Pergamon, Oxford, 1984, p. 157 Search PubMed;
(b) G. H. C. Woo, J. K. Snyder and Z. K. Wan, Prog. Heterocycl. Chem., 2002, 14, 279 CAS;
(c) D. J. Brown, in “The Chemistry of Heterocyclic Compounds”, ed. E. C. Taylor and P. Wipf, John Wiley & Sons, New Jersey, 2004 Search PubMed;
(d) H. R. Darabi, S. Mohandessi, K. Aghapoor and F. Mohsenzadeh, Catal. Commun., 2007, 389 CrossRef CAS;
(e) M. M. Heravi, S. Taheri, K. Bakhtiari and H. A. Oskooie, Catal. Commun., 2007, 211 CrossRef CAS;
(f) S. V. More, M. N. V. Sastry and C.-F. Yao, Green Chem., 2006, 91 RSC;
(g) Z. Zhao, D. D. Wisnoski, S. E. Wolkenberg, W. H. Leister, Y. Wang and C. W. Lindsley, Tetrahedron Lett., 2004, 45, 4873 CrossRef CAS;
(h) R. S. Bhosale, S. R. Sarda, S. S. Ardhapure, W. N. Jadhav, S. R. Bhusare and R. P. Pawar, Tetrahedron Lett., 2005, 46, 7183 CrossRef CAS;
(i) S. V. More, M. N. V. Sastry, C.-C. Wang and C.-F. Yao, Tetrahedron Lett., 2005, 46, 6345 CrossRef CAS.
- For the displacement of chloro group from methyl 2-chloro-1H-indole-3-carboxylate by an oxygen nucleophile e.g. a phenol derivative in the presence NaH that followed an addition–elimination mechanism, see:
(a) K. S. Feldman and D. B. Vidulova, Heterocycles, 2003, 60, 1615 CrossRef CAS . For a similar reaction of N-substituted 2-chloro-1H-indole-3-carbaldehyde with phenol, see: ;
(b) M. F. Comber and C. J. Moody, Synthesis, 1992, 731 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data for all new compounds, and copies of spectra. See DOI: 10.1039/c6ra03556f |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2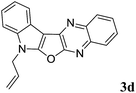
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2