Laser irradiation induced photo-crystallization in nano-structured amorphous Se90−xHgxS10 (x = 0, 5, 10, 15) thin films
Abstract
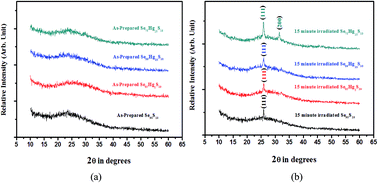
The present study focuses on the influence of laser irradiation induced photo crystallization on the modification of the optical and electrical properties of thermally evaporated amorphous Se90−xHgxS10 (x = 0, 5, 10, 15) thin films. The chemical composition and amorphous nature of the as-prepared thin films were examined by energy dispersive X-ray analysis and X-ray diffraction (XRD) respectively. However, the XRD analysis revealed the crystalline nature of the thin films irradiated using a 337.1 nm pulsed laser. These results were further investigated by surface morphological techniques, namely atomic force microscopy (AFM) and scanning electron microscopy (SEM). An enhancement in the average grain sizes after laser irradiation was observed. The absorption spectra obtained using a UV-vis-spectrophotometer were evaluated using the Urbach’s edge method. As the duration of laser irradiation was increased, the optical energy gap and tail energy width for all the compositions decreased. Further, it has been observed that the value of the optical energy gap decreases with Hg content in the Se–S alloy. These results have been interpreted on the basis of laser irradiation-induced photo-crystallization in the film. Electrical analyses such as dark dc conductivity and photoconductivity in the temperature range 310–390 K show an enhancement of the electrical conductivity and a reduction in activation energy as the irradiation time increases and specify that the density of defect states decreases after irradiation. Temperature dependent photoconductivity measurements show similar trends to that of dark conductivity. This is because the photo-created carriers can move easily in laser irradiated thin films due to the crystallinity of the material.

 Please wait while we load your content...
Please wait while we load your content...