Synthesis, properties and singlet oxygen generation of thiazolidinone double bond linked porphyrin at meso and β-position†
Sohail Ahmad,
Kumar Karitkey Yadav,
Uma Narang,
Soumee Bhattacharya,
Sarangthem Joychandra Singh and
S. M. S. Chauhan*
Bio-organic Research Laboratory, Department of Chemistry, University of Delhi, Delhi-110007, India. E-mail: smschauhan@chemistry.du.ac.in; Fax: +91-11-27666845; Tel: +91-11-27666845 Tel: +91-9871969266
First published on 6th April 2016
Abstract
Meso and β-substituted free base and zinc metallated thiazolidinone–porphyrin conjugates were synthesized by one pot four component Knoevenagel condensation by utilizing substituted amines, carbon disulfide, ethyl chloroacetate and porphyrin aldehydes. These newly synthesized conjugates were characterized by IR, 1H NMR, 13C NMR, UV-Vis, fluorescence and HRMS spectroscopy. The singlet oxygen generation behaviors of these porphyrin conjugates were studied and it was observed that these porphyrin conjugates followed type II singlet oxygen. Fluorescence and singlet oxygen quantum yields among meso-substituted (mono-, di, tetra) and β-substituted conjugates were examined. The photocatalytic photooxidation of naphthols and furan by using these new organic photocatalysts were further analysed and it was observed that meso-tetra substituted (Zn3a) and β-substituted (Zn6a) porphyrins are much efficient for generation of singlet oxygen and for photocatalytic photooxidation.
Introduction
Singlet oxygen (1O2) is one of the most reactive oxygen species (ROS) and holds a prominent role in various chemical and biological processes like medical sciences, photodynamic therapy (PDT),1,2 waste water treatment3 and photooxygenation reactions.4 The extensive applications of 1O2, lead to the development of a series of photosensitizers including xanthene, rosebengal, methylene blue and porphyrin derivatives.4b,5Recently, the porphyrin derivatives have gained a considerable interest due to the presence of their unique photochemical and photophysical properties.6 Among the different classes of the PDT drugs, only porphyrin derivatives are approved by food and drug administration (FDA) for human treatment. Tetraphenylporphyrin (TPP) has been effectively used for the photooxygenation reactions but it possesses certain disadvantages such as poor solubility, low singlet oxygen generation efficiency and photodegradation which limit its biological and photooxygenation applications.7 In porphyrins, the increase in generation of singlet oxygen has been attributed to the high intersystem crossing efficiency8 and is improved either by substitution or metal ion incorporation.9 Moreover, porphyrins associated with electron-donor or electron-acceptor moieties through conjugated system led to the promising PDT results.1
Therefore the synthesis of newer porphyrins, with high singlet oxygen efficiency, photo stability and better solubility is quite challenging for their applications in PDT and photooxygenation reactions.
Rhodanine (thiazolidinone) derivatives have been widely studied as antibacterial, antiviral, antidiabetic agents.10 A variety of rhodanine–porphyrin have been designed and synthesized to modify their binding ability toward proteins and demonstrating their importance as porphyrin-base photosensitizers and photocleaving agents.11 Recently, rhodanine–porphyrin substituted with acid group were found to be good protein probes for proteins as well as their binding affinities and showed promising fluorescence and singlet oxygen quantum yields.11a Rhodanine now become a preferable choice in dyes and solar cell to tune spectroscopic and electrochemical properties by their involvement as electron donor/acceptor agent.12
Herein, we report a new series of meso and β-substituted thiazolidinone porphyrins complexes and investigated the effect of substitution and zinc atom insertion on their intersystem crossing efficiency. The results showed that thiazolidinone–porphyrin conjugates Zn6a and Zn3a exhibit better solubility in aqueous methanolic medium and quantitative yields of singlet oxygen, which makes them an ideal sensitizer for applications in photodynamic therapy and green photooxygenation processes.
Results and discussion
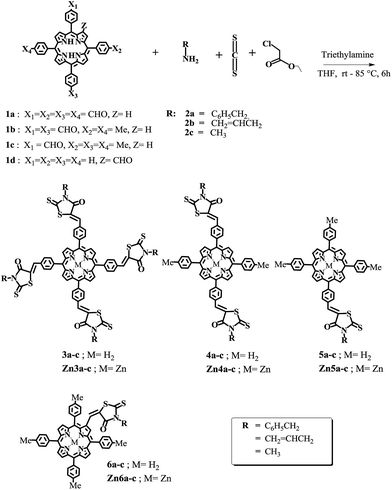
Synthesis of the double bond linked meso-tetra-substituted porphyrin thiazolidinone conjugate 3a was achieved in 36% yield by four-component Knoevenagel condensation of 5,10,15,20-tetrakis(4′-formylphenyl)porphyrin (1a), benzylamine (2a), ethyl chloroacetate and carbon disulfide in the presence of a catalytic amount of triethylamine (TEA) in tetrahydrofuran (THF) at room temperature for 2 hours then followed by 85 °C for 4 hours (Scheme 1). The product was purified by column chromatography on activated neutral aluminum oxide using CHCl3 as an eluent. The same reaction with allylamine (2b) and methylamine (2c) gave 3-allyl-2-thioxo-thiazolidin-4-one (3b) and 3-methyl-2-thioxo-thiazolidin-4-one (3c) porphyrin conjugates in 28% and 36% yield respectively. While, the synthesis of meso-di and mono substituted thiazolidinone linked porphyrins 4a–c and 5a–c were achieved by using 5,15-di(4′-formylphenyl)-10,20-di(4′′-methylphenyl)porphyrin (1b) and 5-(4′-formylphenyl)-10,15,20-tri(4′′-methylphenyl)porphyrin (1c) respectively with primary amine, ethyl chloroacetate and carbon disulfide (CS2) in THF. The thiazolidinone conjugated newer porphyrins (3–5) were well characterized by NMR, UV-Visible, infrared (IR), elemental analysis and mass spectrometry and spectral data were in full agreement with the proposed structures. The infrared absorption spectra of these thiazolidinone conjugated porphyrins (3–5) exhibited a characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1699–1731 cm−1 and the C
O stretching band at 1699–1731 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretching vibration in the region of 1290–1092 cm−1. In 1H NMR spectra, a singlet at δ −2.8 ppm was assigned for the internal NH protons of porphyrins and a singlet in aromatic region at δ 8.61–8.92 ppm was the characteristic signal for β-pyrrolic protons. Further, the presence of a singlet around δ 8.02 ppm was characteristic for vinylic CH proton corresponding to porphyrin–CH
S stretching vibration in the region of 1290–1092 cm−1. In 1H NMR spectra, a singlet at δ −2.8 ppm was assigned for the internal NH protons of porphyrins and a singlet in aromatic region at δ 8.61–8.92 ppm was the characteristic signal for β-pyrrolic protons. Further, the presence of a singlet around δ 8.02 ppm was characteristic for vinylic CH proton corresponding to porphyrin–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) thiazolidinone in 1H NMR spectra, confirmed the attachment of thiazolidinone moiety with porphyrins.
thiazolidinone in 1H NMR spectra, confirmed the attachment of thiazolidinone moiety with porphyrins.
The β-fused porphyrin thiazolidinone conjugates (6a–c) were prepared by condensation of one equivalent of 2-formyl-5,10,15,20-tetra(4′′-methylphenyl)porphyrin (1d) with one equivalents of ethyl chloroacetate and corresponding amine in the presence of CS2 and triethylamine using THF as a solvent at 85 °C for 12 hours. The conjugate were purified by silica gel column chromatography to afford brown solids 6a in 15%, 6b in 11% and 6c in 27% yields respectively. The appearance of vinyl proton at about δ 7.47 ppm as a sharp singlet in the 1H NMR spectra and a corresponding m/z peak in the mass spectra confirmed the formation of 6a–c conjugates.
All the free base thiazolidinone–porphyrin were transformed to corresponding zinc complexes in 91–83% yield by using three equivalent of Zn(OAc)2 in CHCl3–methanol solution. Among these compounds, free base thiazolidinone–porphyrin showed solubility in common organic solvents like chloroform, methanol, and dimethyl sulfoxide (DMSO), whereas zinc complexes showed good solubility in both organic solvents as well as in the aqueous methanolic media.
The absorption spectra of free base conjugates and their corresponding zinc complexes are given in Table 1. On comparing the electronic absorption spectra of mono substituted (5a), di-substituted (4a) and tetra-substituted (3a) conjugates, it was observed that the broadeness and red shiftness of Soret and Q band increases with the increase of thiazolidinone moiety on the porphyrin ring (ESI†) and followed the order of 3a > 4a > 5a. Porphyrin conjugate 3a showed maximum red shifting of 17 nm from simple TPP. When it was compared on the basis of the nature of N-alkylation among tetra-substituted porphyrin, the shifting of Soret band followed the order of 3a > 3b > 3c > TPP. Thus the introduction of thiazolidinone moiety to porphyrin ring greatly changed the energy of singlet excited state. While red shift in the absorption spectra can be induced by the structural distortion of porphyrin π-system as well as by electronic effect of thiazolidinone.
| Compound | Absorptiona λmax/nm | Fluorescencea λem/nm | Φfa | ΦΔa | Quinone (8a)b yieldc (%) |
|---|---|---|---|---|---|
| a Spectra recorded in methanol.b Reaction conditions: napthol 7a (0.10 mmol, 1 equivalent), photocatalyst (0.02 equivalent) in chloroform (5 mL).c Yield of the isolated product 8a. | |||||
| 3a | 431.1, 521.6, 559.9, 597.3, 650.9 | 670, 734 | 0.076 | — | — |
| Zn3a | 430.9, 555.4, 595.9 | 680 | 0.023 | 0.83 ± 0.03 | 82 |
| 3b | 424.5, 518.5, 560.2, 591.2, 654.3 | 666, 725 | 0.093 | — | — |
| Zn3b | 423.7, 549.8, 578 | 616, 649 | 0.028 | 0.75 ± 0.02 | 64 |
| 3c | 421.3, 512.3, 551.7, 589.3, 651.9 | 660, 722 | 0.097 | — | — |
| Zn3c | 420.8, 548.8, 576.1 | 615, 665 | 0.031 | 0.72 ± 0.02 | 63 |
| 5a | 419.1, 516.2, 550.0, 590.8, 651.2 | 652, 711 | 0.089 | — | — |
| Zn5a | 420.1, 548.3 | 610, 648 | 0.029 | 0.72 ± 0.03 | 60 |
| 4a | 422.3, 517.4, 551.7, 591.3, 651.8 | 658, 712 | 0.084 | — | — |
| Zn4a | 420.8, 550.2 | 613, 652 | 0.027 | 0.78 ± 0.03 | 66 |
| 6a | 433.2, 456.8, 528.7, 580.9, 615.4 | 701, 753 | 0.062 | — | — |
| Zn6a | 466.5, 564.3, 614.3 | 706 | 0.015 | 0.89 ± 0.02 | 87 |
The free base β-substituted porphyrin (6a) showed different electronic absorption spectrum than meso-substituted porphyrins (ESI†) and appeared as very broad Soret and Q bands. The UV-Visible spectra of 6a showed splitting of Soret band at 433 and 456 nm, while Q bands appeared at 528, 580, 615 and 667 nm. Further the zinc metallated β-porphyrin Zn6a did not show the splitting of Soret band and appeared at 466 nm along with the Q bands at 564, 614 nm (ESI†). The β-substituted porphyrins 6a and Zn6a contains the direct involvement of conjugation of thiazolidinone with the 18π-molecular system of porphyrin ring, resulting in the substantial perturbation of the photophysical and electrochemical properties of the porphyrin.9 This perturbation can be mainly ascribed to the conjugation of π-orbitals from both the linker and the pyrrolic ring which may induce some charge transfer character to porphyrin π → π* transitions that are no longer totally centred on the porphyrin ring.13
The emission spectra of the 3a (671 and 734 nm) in methanol are quite broadened and red-shifted compared to 3b (666 and 725 nm) and 3c (660 and 722 nm), Table 1 (ESI†). The red shifts in porphyrins suggests that there is a increment in the π-conjugation of these molecules which results in the reduction of the energy gap between highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO).14 Further the emission spectrum of β-substituted porphyrin 6a exhibit very broad emission at 701 and 753 nm on excitation at 440 nm while the corresponding zinc complex Zn6a showed good emission peak at 706 nm.
The fluorescence quantum yields (Φf) of thiazolidinone–porphyrin conjugates were examined from the emission and absorption spectra by a comparative method9,15 (Table 1). From the results, it is observed that the quantum yields are considerably reduced on attachment of thiazolidinone moiety at meso and β-positions of porphyrins. The reasons behind the reduced fluorescence quantum yields are the enhanced non-radiative decay rates of the internal conversion and/or intersystem crossing.15,16 Thiazolidinone substituents are mainly responsible for the enhancement in intersystem crossing rates, thus causing reduced fluorescence quantum yields. Free base porphyrin showed high fluorescence quantum yields (Φf) whereas the corresponding zinc complex showed decrease in fluorescence quantum yields (Φf). The fluorescence quantum yields of different tetra substituted zinc conjugates followed the order of Zn3c (Φf = 0.031) > Zn3b (Φf = 0.028) > Zn3a (Φf = 0.023) and among mono, di and tetra substituted zinc conjugates followed the order of Zn5a (Φf = 0.023) > Zn4a (Φf = 0.027) > Zn3a (Φf = 0.023). The β-substituted porphyrin conjugate Zn6a showed much lower fluorescence quantum yields (Φf = 0.015) compared to other thiazolidinone–porphyrin conjugates.
An EPR spin trapping technique was used for the identification of reactive oxygen species (ROS). The oxygen saturated acetonitrile solution of 3a (0.1 mM) and 2,2,6,6-tetrmethylpiperidine (TEMP) (50 mM) were irradiated with a 355 nm laser, and a three-line EPR spectrum with equal intensity and a hyperfine coupling constant of aN = 15.9 G was observed, which could be assigned to TEMPO (TEMP-1O2 adduct).17 However, when 5,5-dimethyl-1-pyrroline N-oxide (DMPO) was used as the spin trapping agent for superoxide anion radicals and hydroxyl radicals, no EPR signals were observed (even in the presence of water, a condition favourable for the observation of the DMPO–OH adduct signal). These results indicate that 3a showed 1O2-based or type II photodynamic activities.
Singlet oxygen efficiency of zinc thiazolidinone–porphyrin conjugates was qualitatively evaluated by the photodecomposition of 1,3-diphenylisobenzofuran (DPiBF)18–21 in the presence of zinc thiazolidinone–porphyrin conjugates (Table 1). Methanolic solution of zinc thiazolidinone–porphyrin conjugates and DPiBF was irradiated with a 200 W mercury lamp over a time period of 0–60 min. The peak corresponding to DPiBF showed decrease in absorbance in UV-Vis spectra. The singlet oxygen quantum yields were estimated by plotting the depletion in absorbance of DPiBF against the irradiation time using 5,10,15,20-tetraphenyl porphyrin zinc (ZnTPP) as a reference. Zn6a (0.89 ± 0.02) and Zn3a (0.83 ± 0.03) showed larger value of 1O2 quantum yield, as compared to other zinc thiazolidinone–porphyrin conjugates (Table 1). The di and mono substituted zinc complexes (Zn4a and Zn5a) showed value of 0.78 ± 0.03 and 0.72 ± 0.03 respectively.
The photoreactions of naphthol (7a) were studied and its 1O2 generation efficiencies were compared among different conjugates. Reactions were carried out in O2 atmosphere (1 atm), 25 °C using 200 W mercury lamp and the reaction was monitored the UV-Vis changes. Naphthol 7a reacts with singlet oxygen to generate the quinone 8a. Different zinc substituted thiazolidinone–porphyrin conjugates showed different singlet oxygen generation efficiency and thus gave different yields of quinone (Table 1). The reaction show excellent when Zn6a and Zn3a used as the catalyst for the oxidative reaction (Table 1) and the reaction was completed within one hour. Zn6a and Zn3a gave 8a in 87% and 82% yield respectively. Furthermore, when mono substituted and di-substituted conjugates used as photocatalyst, slow conversion was observed and gave 8a in 60% and 66% yield respectively. The scope and efficiency of Zn6a in oxidative reaction was further explored by different naphthol derivatives as well we also used less activated substrates such as furan and the result are summarized in Table 2.
Conclusion
In conclusion, we have synthesized and characterized a range of newer meso and β-substituted thiazolidinone and porphyrin conjugates. Their fluorescence and singlet oxygen quantum yields were determined by various techniques. These porphyrin conjugates showed red shift of their UV-Vis spectra as compared with simple TPP. The β-substituted zinc thiazolidinone–porphyrin conjugate showed better singlet oxygen generation efficiency than other thiazolidinone–porphyrin conjugates.Experimental section
General
All reactions were performed under a nitrogen atmosphere. Pyrrole and aromatic aldehydes were purchased from Aldrich and used without further purification. Solvents were purchased from Merck and dried according to literature prior to use. Reactions were monitored by thin layer chromatography (TLC) and products were purified by column chromatography using activated neutral aluminum oxide. 1H NMR spectra were recorded in CDCl3 using a Jeol 400 MHz NMR spectrometer. Chemical shifts are expressed in parts per million (ppm) relative to tetramethylsilane (TMS, 0 ppm) as an internal standard. Coupling constants (J) are reported in Hertz (Hz). 13C NMR spectra were recorded on Jeol 100 MHz NMR spectrophotometer. Infrared spectra were recorded on a Perkin Elmer IR spectrometer and absorption maxima are given in cm−1. A Perkin Elmer UV-Vis spectrophotometer was used for UV measurements. The fluorescence spectra were obtained by using a Shimadzu RF-5301PC spectrofluorophotometer. The fluorescence quantum yields of these compounds were determined by comparing to a calibration standard of TPP in benzene solution with a fluorescence quantum yield, of 0.11 (different refractive indices of the solvents used in the standard and sample were corrected).General procedure for the synthesis of porphyrinic rhodanine derivative
In a 25 mL round bottom flask, primary amine (5 mmol), CS2 (8 mmol), ethyl chloroacetate (5 mmol), porphyrin aldehyde (1 mmol) and catalytic amount of triethylamine (0.4 mmol) in THF (5 mL) were mixed under nitrogen atmosphere and the reaction was stirred at 80 °C for the appropriate time. The reaction was monitored by TLC. After completion of the reaction, the mixture was poured into the water and the products were extracted with ethyl acetate. The organic layer was separated, washed with 2–3 times with water, dried over anhydrous sodium sulfate and evaporated. The crude product was purified by column chromatography on neutral alumina using 30% ethyl acetate (EtOAc) in hexane as an eluent to give rhodanine conjugated porphyrins in 15–18%.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.84–7.82 (d, J = 8.1 Hz, 8H, meso-ArH), 7.48–7.47 (d, J = 7.8 Hz, 8H, benzyl-ArH), 7.33–7.27 (m, 12H, benzyl-ArH), 5.34 (s, 8H, CH2), −2.84 (s, 2H, internal NH) ppm; 13C NMR (100 MHz, CDCl3): δ = 43.51, 115.24, 118.16, 120.38, 124.53, 125.84, 126.48, 140.08, 142.90, 145.92, 170.29, 196.42 ppm.
C), 7.84–7.82 (d, J = 8.1 Hz, 8H, meso-ArH), 7.48–7.47 (d, J = 7.8 Hz, 8H, benzyl-ArH), 7.33–7.27 (m, 12H, benzyl-ArH), 5.34 (s, 8H, CH2), −2.84 (s, 2H, internal NH) ppm; 13C NMR (100 MHz, CDCl3): δ = 43.51, 115.24, 118.16, 120.38, 124.53, 125.84, 126.48, 140.08, 142.90, 145.92, 170.29, 196.42 ppm.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.64–7.62 (d, J = 8.1 Hz, 8H, meso-ArH), 5.78 (m, 4H, CH), 5.28 (m, 8H, CH2), 4.53 (s, 8H, CH2), −2.821 (s, 2H, internal NH) ppm.
C), 7.64–7.62 (d, J = 8.1 Hz, 8H, meso-ArH), 5.78 (m, 4H, CH), 5.28 (m, 8H, CH2), 4.53 (s, 8H, CH2), −2.821 (s, 2H, internal NH) ppm.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 3.44 (s, 12H, CH3), −2.80 (s, 2H, internal NH) ppm.
C), 3.44 (s, 12H, CH3), −2.80 (s, 2H, internal NH) ppm.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.82–7.80 (d, J = 8.1 Hz, 8H, meso-ArH), 7.46–7.44 (d, J = 7.8 Hz, 8H, benzyl-ArH), 7.31–7.24 (m, 12H, benzyl-ArH), 5.29 (s, 8H, CH2) ppm.
C), 7.82–7.80 (d, J = 8.1 Hz, 8H, meso-ArH), 7.46–7.44 (d, J = 7.8 Hz, 8H, benzyl-ArH), 7.31–7.24 (m, 12H, benzyl-ArH), 5.29 (s, 8H, CH2) ppm.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.61–7.59 (d, J = 8.1 Hz, 8H, meso-ArH), 5.75 (m, 4H, CH), 5.23 (m, 8H, CH2), 4.52 (s, 8H, CH2).
C), 7.61–7.59 (d, J = 8.1 Hz, 8H, meso-ArH), 5.75 (m, 4H, CH), 5.23 (m, 8H, CH2), 4.52 (s, 8H, CH2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 3.41 (s, 12H, CH3).
C), 3.41 (s, 12H, CH3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 7.80–7.87 (d, J = 8.4 Hz, 2H, meso-ArH), 7.89 (m, 2H, ArH), 7.87 (m, 3H, ArH), 5.38 (s, 2H, CH2), 2.50 (d, 9H, CH3), −2.83 (s, 2H, internal NH) ppm; CHN: obtained C, 78.16; H, 4.50; N, 6.98; S, 7.51 (calculated C, 78.26; H, 4.87; N, 7.87; S, 7.20).
C), 7.80–7.87 (d, J = 8.4 Hz, 2H, meso-ArH), 7.89 (m, 2H, ArH), 7.87 (m, 3H, ArH), 5.38 (s, 2H, CH2), 2.50 (d, 9H, CH3), −2.83 (s, 2H, internal NH) ppm; CHN: obtained C, 78.16; H, 4.50; N, 6.98; S, 7.51 (calculated C, 78.26; H, 4.87; N, 7.87; S, 7.20).Photooxidation of naphthols to naphthoquinone
Photocatalyst (0.02 equivalent) was added to a stirred solution of naphthol (0.5 mmol) in methanol (5 mL). The reaction mixture was stirred at 25 °C under air atmosphere. The solution was then irradiated using a 200 W mercury lamp. LCMS and TLC were used to monitor the progress of the reaction. After the reaction is completed, the solvent was evaporated under reduced pressure. The mixture was purified by column chromatography.Acknowledgements
This research work was financially supported by Department of Science and Technology (DST) New Delhi, DU-DST Purse grant and University of Delhi. K. K. Yadav and S. Bhattacharya are thankful to UGC New Delhi and U. Narang is thankful to CSIR New Delhi for their senior research fellowships.Notes and references
- J. Schmitt, V. Heitz, A. Sour, F. Bolze, H. Ftouni, J.-F. Nicoud, L. Flamigni and B. Ventura, Angew. Chem., Int. Ed., 2015, 54, 169–173 CrossRef CAS PubMed.
- (a) M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC; (b) R. Bonnett, Chem. Soc. Rev., 1995, 24, 19–33 RSC.
- A. G. Griesbeck, J. Uhlig, T. Sottmann, L. Belkoura and R. Strey, Chem.–Eur. J., 2012, 18, 16161–16165 CrossRef CAS PubMed.
- (a) M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351–371 CrossRef CAS; (b) B. Fuckel, D. A. Roberts, Y. Y. Cheng, R. G. C. R. Clady, R. B. Piper, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, J. Phys. Chem. Lett., 2011, 2, 966–971 CrossRef CAS.
- H. Abramczyk, B. Brozek-Pluska, M. Tondusson and E. Freysz, J. Phys. Chem. C, 2013, 117, 4999–5013 CAS.
- A. O. Ribeiro, J. P. C. Tome, M. G. P. M. S. Neves, A. C. Tome, J. A. S. Cavaleiro, Y. Iamamoto and T. Torres, Tetrahedron Lett., 2006, 47, 9177–9180 CrossRef CAS.
- R. Bonnett, B. D. Djelal and A. Nguyen, J. Porphyrins Phthalocyanines, 2001, 5, 652–661 CrossRef CAS.
- S. Silva, P. M. R. Pereira, P. Silva, F. A. A. Paz, M. A. F. Faustino, J. A. S. Cavaleiroa and J. P. C. Tome, Chem. Commun., 2012, 48, 3608–3610 RSC.
- Z. Valicseka, O. Horvátha and K. Patonay, J. Photochem. Photobiol., A, 2011, 226, 23–35 CrossRef.
- J. F. Lovell, T. W. B. Liu, J. Chen and G. Zheng, Chem. Rev., 2010, 110, 2839–2857 CrossRef CAS PubMed.
- H. M. Wang, J. Q. Jiang, J. H. Xiao, R. L. Gao, F. Y. Lin and X. Y. Liu, Chem.-Biol. Interact., 2008, 172, 154–158 CrossRef CAS PubMed.
- B. Marydasan, A. K. Nair and D. Ramaiah, J. Phys. Chem. B, 2013, 117, 13515–13522 CrossRef CAS PubMed.
- A. Lembo, P. Tagliatesta and D. M. Guldi, J. Phys. Chem. A, 2006, 110, 11424–11434 CrossRef CAS PubMed.
- W.-Z. Ke, L. Wang, B. Song, J.-X. Yang, Y.-P. Tian, Y.-H. Kan, X.-T. Tao and M.-H. Jiang, Inorg. Chem. Commun., 2011, 14, 1311–1313 CrossRef CAS.
- E. Annoni, M. Pizzotti, R. Ugo, S. Quici, T. Morotti, M. Bruschi and P. Mussini, Eur. J. Inorg. Chem., 2005, 3857–3874 CrossRef CAS.
- J. A. Shelnutt and V. Ortiz, J. Phys. Chem., 1985, 89, 4733–4739 CrossRef CAS.
- N. C. Maiti and M. Ravikanth, J. Chem. Soc., Faraday Trans., 1996, 92, 1095–1100 RSC.
- I. Gupta and M. Ravikanth, Inorg. Chim. Acta, 2007, 360, 1731–1742 CrossRef CAS.
- I. Gupta and M. Ravikanth, J. Chem. Sci., 2005, 117, 161–166 CrossRef CAS.
- Y. Lion, M. Delmelle and A. V. D. Vorst, Nature, 1976, 263, 442–443 CrossRef CAS PubMed.
- Z. Zeng, J. Zhou, Y. Zhang, R. Qiao, S. Xia, J. Chen, X. Wang and B. Zhang, J. Phys. Chem. B, 2007, 111, 2688–2696 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra of all new compounds. See DOI: 10.1039/c6ra03489f |
| This journal is © The Royal Society of Chemistry 2016 |