How does an amalgamated Ni cathode affect carbon nanotube growth? A density functional theory study
Abstract
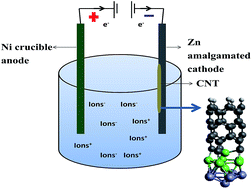
This is a Density Functional Theory (DFT) study on the influence of an alloying mixture of Ni–Zn catalysts on carbon nanotube, CNT, growth. The study is inspired by the one pot synthesis of carbon nanofibers during the electrolysis of Li2CO3. Unlike CVD, CNT growth initiates at the liquid/solid, rather than gas/solid interface in the above process. The electrodes are an amalgamated Zn cathode and with a pure Ni crucible as the anode, and both zinc and nickel (or other transition metals) are required for high yield production. The use of transition metals as the catalyst for CNT, CVD growth is well known. However, in this study we show how a mixture of the Zn–Ni alloy can act as the catalyst for the effective CNT growth. Ni and Zn are taken as an example of first row transition metals with a partially empty and completely filled d orbital respectively. The study shows that the π–d bonding between the nanotube and the metal results in strong bond formation at the interface of the nanotube growth. The study remains valid for other such metal alloys with partially and completely filled d orbitals.

 Please wait while we load your content...
Please wait while we load your content...