Investigations of the band structures of edge-defect zigzag graphene nanoribbons using density functional theory†
Abstract
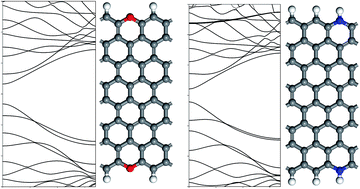
We used density functional theory to investigate the band gap and the electron conductivity of edge-defect zigzag graphene nanoribbons (ZGNR). The band structures of edge-oxidized ZGNRs (ox-ZGNRs) were hardly affected by introducing carboxyl and hydroxyl groups, but were markedly altered near the Fermi level by the ketone and ether groups, resulting in open band gaps of 0.6064 eV and 0.6413 eV, respectively. Unlike carboxyl and hydroxyl groups, the ketone and ether groups caused noticeable changes due to disruption of the sp2 hybridization of edge carbon atoms via forming chemical bonds with them. While the band gaps for both-side pyridinic and pyrrolic edge-nitrided ZGNRs (N-ZGNRs) holding aromatic forms are respectively calculated to 0.2882 eV and 0.3057 eV, the non-aromatic graphitic group has a higher band gap of 0.4244 eV. The edge-aromatic ZGNRs including carboxyl or hydroxyl groups in ox-ZGNRs and pyridinic or pyrrolic groups in N-ZGNRs show similar features of band structures and properties as displayed in a pristine ZGNR. On the contrary, the edge-nonaromatic ZGNRs modified with ketone, ether, or graphitic groups have different shapes of the band structures whose band gaps are more open than that of a pristine ZGNR.

 Please wait while we load your content...
Please wait while we load your content...