Conductive and SERS-active colloidal gold films spontaneously formed at a liquid/liquid interface†
Abstract
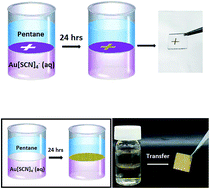
We show that water-soluble gold thiocyanate salt [KAu(SCN)4] spontaneously formed electrically-conductive and surface enhanced Raman scattering (SERS)-active films at the interface between water and pentane. Microscopy and spectroscopic analyses reveal that the films comprised of condensed Au nanoparticles, assembled via crystallization of KAu(SCN)4 and self-reduction of the Au3+ ions by the thiocyanate ligands. Importantly, the pentane phase was crucial in promoting the crystallization/reduction reactions. The Au films exhibited useful applications, including excellent conductivity and SERS sensing. We also show that the new film self-assembly process could be readily harnessed for producing macro-scale Au patterns.

 Please wait while we load your content...
Please wait while we load your content...