Electrodeposition of Au nanoparticles on poly(diallyldimethylammonium chloride) functionalized reduced graphene oxide sheets for voltammetric determination of nicotine in tobacco products and anti-smoking pharmaceuticals†
Abstract
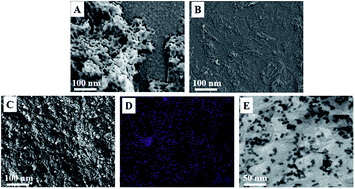
The principal objective of this study was to develop a sensitive and selective electrochemical sensor for nicotine detection based on a novel poly(diallyldimethylammonium chloride) functionalized reduced graphene oxide–gold nanoparticle (PDDA-RGO/Au) nanocomposite. The PDDA-RGO/Au nanocomposite was synthesized by a wet chemical approach followed by an electrodeposition process. The synthesized nanocomposite was characterized by FTIR, Raman spectroscopy, SEM and XRD. The sensor showed electrocatalytic activity in both aqueous and micellar media toward the oxidation of nicotine in Britton–Robinson buffer solution using cyclic voltammetry and electrochemical impedance spectroscopy (EIS) techniques. The results showed that the PDDA-RGO/Au nanocomposite could greatly enhance the electrochemical oxidation of nicotine. The linear response range of the sensor was between 0.5 μM and 300 μM with a detection limit of 0.12 μM. Excellent results were achieved for the determination of nicotine in tobacco products and anti-smoking pharmaceuticals.

 Please wait while we load your content...
Please wait while we load your content...